Pharmazone Walk-in CRA, GMP, PV, QA, QC

- Company Overview
- Job Role & Responsibilities
- GCP – Clinical Research
- GMP – Quality & Operations
- Pharmacovigilance (PV)
- Accounts
- Business Development (BD)
- Eligibility / Qualifications
- Experience Summary
- Location & Salary
- Application Process
- Why Build Your Career with Pharmazone
- Frequently Asked Questions (FAQs)
- Is this walk-in interview open to freshers?
- Can candidates from outside Ahmedabad apply?
- Is prior pharma experience mandatory for all roles?
- How many total vacancies are available?
- Is registration mandatory before walk-in?
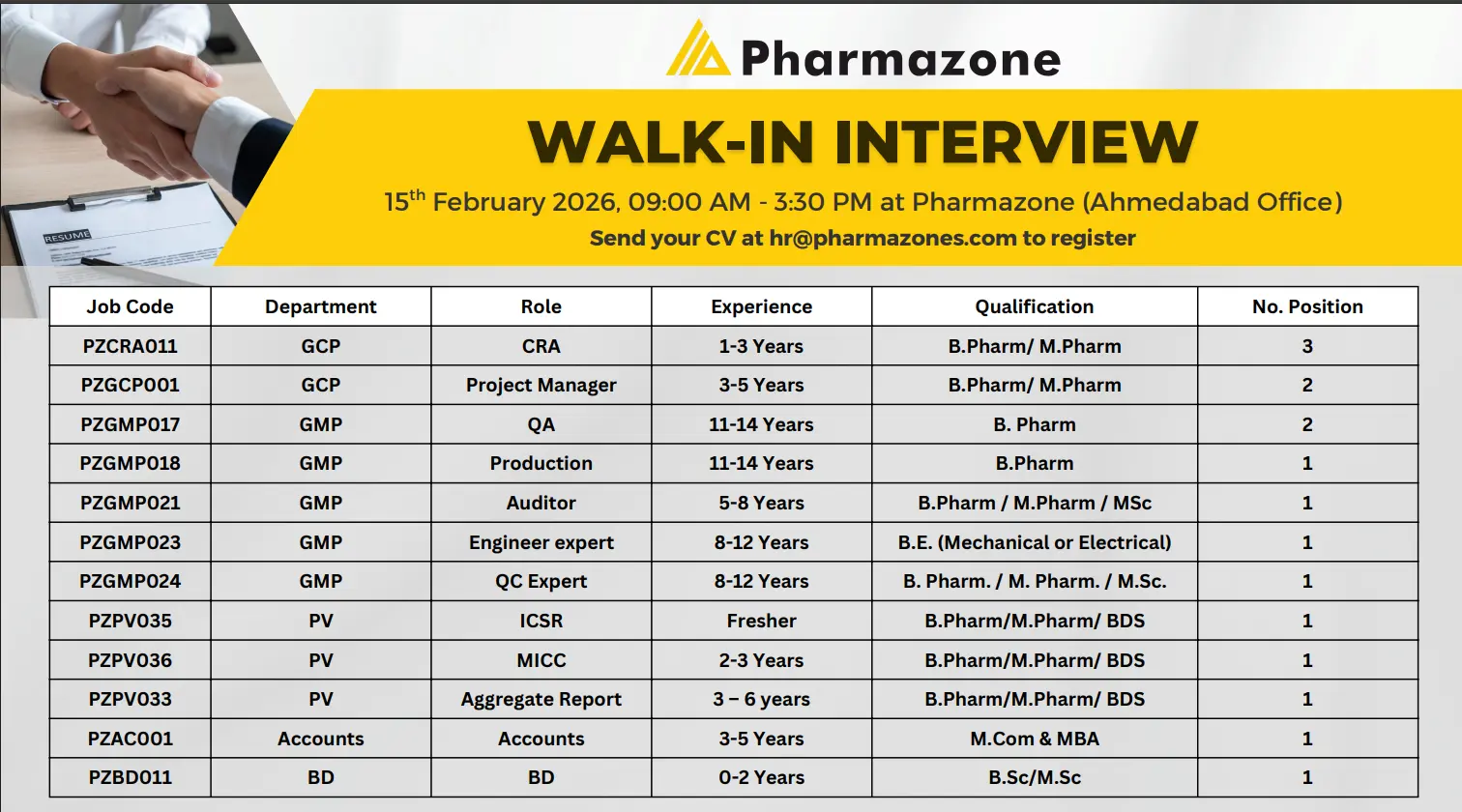
- Summary Table
B.Pharm/M.Pharm 15 Vacancies – Pharmazone Ahmedabad
Pharmazone hiring 15 professionals in CRA, GMP, PV, QA, QC at Ahmedabad. B.Pharm, M.Pharm, MSc eligible. Walk-in 15 Feb 2026.
Pharmazone is conducting a large walk-in interview drive in Ahmedabad for professionals and freshers across clinical research, GCP, GMP, pharmacovigilance, quality, engineering, accounts, and business development functions. This hiring initiative is aimed at strengthening Pharmazone’s regulated services portfolio, supporting pharmaceutical companies, CROs, and life sciences organizations with compliance-driven expertise.
This walk-in drive offers opportunities for both early-career candidates and experienced professionals looking to work in high-demand pharmaceutical domains such as clinical research, regulatory compliance, GMP auditing, pharmacovigilance, and quality systems.
Company Overview
Pharmazone is a specialized pharmaceutical and life sciences services organization providing end-to-end support across clinical research, GCP compliance, GMP consulting, pharmacovigilance, quality assurance, and regulatory operations. The company works closely with pharmaceutical manufacturers, CROs, and healthcare organizations to ensure compliance with global regulatory standards.
With strong domain expertise and a growing client base, Pharmazone has positioned itself as a trusted partner for regulatory audits, clinical operations, safety monitoring, and quality system implementation. The organization emphasizes professional ethics, regulatory accuracy, and continuous learning, making it a strong workplace for professionals seeking long-term careers in pharma and clinical research services.
Job Role & Responsibilities
Pharmazone is hiring across multiple departments. Each role contributes directly to regulatory compliance, patient safety, and pharmaceutical quality assurance.
GCP – Clinical Research
Clinical Research Associate (CRA)
- Site monitoring activities in compliance with GCP guidelines
- Source data verification and site documentation review
- Coordination with investigators and study teams
- Support for clinical trial monitoring and reporting
GCP Project Manager
- End-to-end management of clinical research projects
- Resource planning, timelines, and budget control
- Client communication and study progress reporting
- Ensuring GCP compliance across study activities
GMP – Quality & Operations
Quality Assurance (QA)
- Implementation and maintenance of GMP quality systems
- Handling deviations, CAPA, change controls, and investigations
- Support during regulatory and client audits
Production
- Oversight of GMP-compliant production activities
- Documentation and batch record review
- Coordination with QA and engineering teams
GMP Auditor
- Planning and execution of GMP audits
- Audit reporting and follow-up actions
- Vendor qualification and compliance assessments
Engineer Expert
- Engineering support for GMP facilities
- Equipment qualification and maintenance
- Utilities compliance and documentation
Quality Control (QC) Expert
- Analytical oversight and laboratory compliance
- Review of QC data and investigation of OOS/OOT
- Support for regulatory inspections
Pharmacovigilance (PV)
ICSR (Fresher)
- Case processing and safety data entry
- Adverse event reporting as per regulatory timelines
MICC
- Medical information case management
- Literature screening and safety reporting
Aggregate Report
- Preparation of PSUR, PBRER, and aggregate safety reports
- Signal detection and benefit-risk analysis support
Accounts
- Financial accounting and reporting
- Compliance with statutory and internal controls
Business Development (BD)
- Client acquisition and relationship management
- Support for pharma and CRO business expansion
Eligibility / Qualifications
Eligibility varies by role. Detailed criteria are listed below:
Relevant Courses (Comma-Separated):
B.Pharmacy, M.Pharmacy, M.Sc, BDS, Life Sciences, Clinical Research, Mechanical Engineering, Electrical Engineering, M.Com, MBA, B.Sc, M.Sc Biotechnology
Experience Summary
- CRA: 1–3 years
- GCP Project Manager: 3–5 years
- QA / Production (GMP): 11–14 years
- GMP Auditor: 5–8 years
- Engineer Expert: 8–12 years
- QC Expert: 8–12 years
- PV ICSR: Fresher
- PV MICC: 2–3 years
- PV Aggregate Report: 3–6 years
- Accounts: 3–5 years
- Business Development: 0–2 years
Location & Salary
Interview Location:
Pharmazone – Ahmedabad Office
Interview Date & Time:
15 February 2026
09:00 AM to 03:30 PM
Salary & Compensation:
Salary will be offered as per industry standards and will depend on role, experience, and domain expertise. Pharmazone offers competitive CTC packages aligned with pharmaceutical and CRO industry benchmarks.
Application Process
Candidates are required to register by sharing their CV before attending the walk-in interview.
Register via Email:
hr@pharmazones.com
Mention the Job Code in the subject line for faster shortlisting.
Why Build Your Career with Pharmazone
- Exposure to high-value pharma and clinical research projects
- Opportunities across GCP, GMP, and pharmacovigilance domains
- Strong learning environment for freshers and experienced professionals
- Work with regulated markets and global compliance standards
- Stable growth-driven organization in pharma services
Frequently Asked Questions (FAQs)
Is this walk-in interview open to freshers?
Yes. Fresher roles are available in Pharmacovigilance (ICSR) and Business Development.
Can candidates from outside Ahmedabad apply?
Yes. Candidates from any location can attend the walk-in interview after CV registration.
Is prior pharma experience mandatory for all roles?
No. Experience requirements vary by position. Please refer to role-specific criteria.
How many total vacancies are available?
There are approximately 15 openings across multiple departments.
Is registration mandatory before walk-in?
Yes. Candidates must email their CV to hr@pharmazones.com to register.
Summary Table
| Category | Details |
|---|---|
| Company | Pharmazone |
| Vacancies | CRA – 3, GCP Project Manager – 2, QA – 2, Production – 1, GMP Auditor – 1, Engineer Expert – 1, QC Expert – 1, PV ICSR – 1, PV MICC – 1, PV Aggregate – 1, Accounts – 1, BD – 1 |
| Required Education | B.Pharm, M.Pharm, M.Sc, BDS, BE, M.Com, MBA |
| Experience | Fresher to 14 years depending on role |
To apply for this job email your details to hr@pharmazones.com


