Piramal Walk-in Qc Qa

- Company Overview
- Job Role & Responsibilities
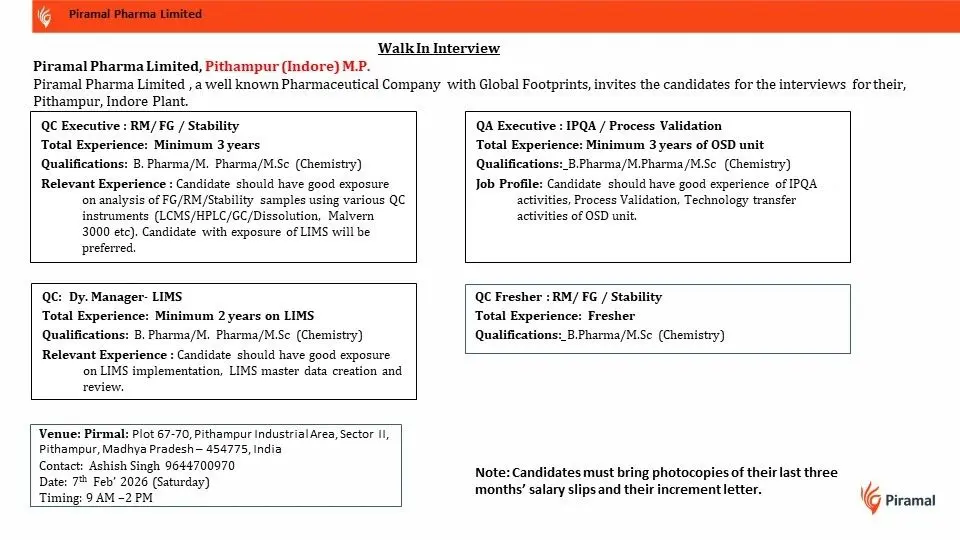
- Quality Control Executive – RM / FG / Stability
- QC Deputy Manager – LIMS
- Quality Assurance Executive – IPQA / Process Validation
- QC Fresher – RM / FG / Stability
- Eligibility / Qualifications
- Location & Salary
- Walk-In Interview Details
- Application Process
- Why Join Piramal Pharma Limited
- Frequently Asked Questions (FAQs)
B.Pharm/M.Pharm QC QA Walk-In – Piramal Pharma Indore
Piramal Pharma hiring QC & QA professionals in Indore. B.Pharm/M.Pharm/M.Sc candidates. Multiple vacancies. Walk-in on 7 Feb 2026.
Piramal Pharma Limited is conducting a walk-in interview at its Pithampur (Indore) manufacturing facility for Quality Control and Quality Assurance professionals. This opportunity is ideal for experienced pharma professionals as well as freshers looking to begin their careers with a globally recognized pharmaceutical company known for compliance-driven manufacturing and innovation-led healthcare solutions.
With a strong presence across regulated markets, Piramal Pharma offers structured career growth, exposure to advanced analytical technologies, and hands-on involvement in GMP-compliant operations. The walk-in drive is scheduled for 7th February 2026 and covers multiple QC and QA roles across RM, FG, Stability, IPQA, Process Validation, and LIMS functions.
Company Overview
Piramal Pharma Limited is a globally integrated pharmaceutical company with operations spanning North America, Europe, and emerging markets. The company operates across complex generics, APIs, contract development and manufacturing (CDMO), and critical care segments. Known for its regulatory excellence, Piramal Pharma consistently meets USFDA, EU-GMP, WHO-GMP, and other international compliance standards.
The Pithampur facility near Indore is a key manufacturing hub with state-of-the-art laboratories, validated production blocks, and advanced quality systems. Employees here gain exposure to international audits, data integrity practices, and modern analytical instrumentation, making it a preferred workplace for quality professionals.
Job Role & Responsibilities
Quality Control Executive – RM / FG / Stability
- Perform routine and non-routine analysis of raw materials, finished goods, and stability samples
- Operate and maintain advanced QC instruments including HPLC, LCMS, GC, Dissolution, UV, IR, and particle size analyzers (Malvern 3000)
- Ensure adherence to analytical SOPs, pharmacopeial standards, and regulatory guidelines
- Handle OOS, OOT, deviations, and investigation documentation
- Support stability studies and trending as per ICH guidelines
- Maintain laboratory data integrity and compliance with ALCOA+ principles
- Work closely with QA for batch release and audit readiness
QC Deputy Manager – LIMS
- Lead LIMS implementation, validation, and lifecycle management
- Create, review, and approve LIMS master data and workflows
- Ensure seamless integration of analytical data with QC operations
- Train QC teams on LIMS usage and compliance requirements
- Handle audit queries related to electronic data systems
- Coordinate with IT and QA for system upgrades and change control
Quality Assurance Executive – IPQA / Process Validation
- Perform in-process quality assurance activities across OSD manufacturing
- Review Batch Manufacturing Records (BMR) and Batch Packing Records (BPR)
- Execute and review process validation and cleaning validation protocols
- Support technology transfer activities for new products
- Monitor line clearance, critical process parameters, and GMP compliance
- Handle deviations, change controls, CAPA, and risk assessments
- Ensure audit readiness for internal and external inspections
QC Fresher – RM / FG / Stability
- Assist senior analysts in routine laboratory testing
- Learn operation of analytical instruments under supervision
- Support sample management, documentation, and data entry
- Gain exposure to GMP laboratory practices and compliance systems
Eligibility / Qualifications
Required educational background includes:
- B.Pharm, M.Pharm
- B.Sc Chemistry, M.Sc Chemistry
Relevant courses applicable:
B.Pharm, M.Pharm (Pharmaceutical Analysis, Quality Assurance), B.Sc Chemistry, M.Sc Chemistry (Analytical Chemistry, Organic Chemistry, Physical Chemistry)
Experience requirements:
- QC Executive: Minimum 3 years of relevant experience
- QC Dy. Manager – LIMS: Minimum 2 years of hands-on LIMS experience
- QA Executive: Minimum 3 years of OSD unit experience
- QC Fresher: Fresh graduates eligible
Location & Salary
Job Location: Piramal Pharma Limited, Pithampur Industrial Area, Indore, Madhya Pradesh
Salary: Best in industry, commensurate with experience and role. Detailed CTC discussion during interview.
Walk-In Interview Details
- Date: 7th February 2026 (Saturday)
- Time: 9:00 AM to 2:00 PM
- Venue: Plot No. 67–70, Pithampur Industrial Area, Sector II, Pithampur, Madhya Pradesh – 454775
- Contact Person: Ashish Singh – 9644700970
Application Process
Interested candidates can attend the walk-in interview directly at the venue.
Candidates unable to attend may share their updated CV via official Piramal Pharma recruitment channels when available.
Documents to carry:
- Updated resume
- Photocopies of last three months’ salary slips (for experienced candidates)
- Latest increment letter
- Educational certificates
- Government-issued ID proof
Why Join Piramal Pharma Limited
- Exposure to global regulatory audits
- Strong learning curve in QC and QA domains
- Advanced analytical and quality systems
- Structured career growth and internal mobility
- High standards of data integrity and compliance
Frequently Asked Questions (FAQs)
Is this walk-in interview open to freshers?
Yes, QC Fresher positions are open for eligible B.Pharm and M.Sc Chemistry graduates.
Is experience mandatory for QA roles?
Yes, QA Executive roles require a minimum of 3 years of OSD unit experience.
What type of QC experience is preferred?
Hands-on experience with HPLC, LCMS, GC, Dissolution, Stability studies, and LIMS is highly preferred.
Is LIMS experience compulsory for the Dy. Manager role?
Yes, prior experience in LIMS implementation and master data management is mandatory.
Will salary be discussed on the same day?
Yes, shortlisted candidates can expect initial salary discussions during the interview.
| Company | Piramal Pharma Limited |
|---|---|
| Vacancies | QC Executive (RM/FG/Stability), QC Dy. Manager – LIMS, QA Executive (IPQA/Process Validation), QC Fresher |
| Required Education | B.Pharm, M.Pharm, B.Sc Chemistry, M.Sc Chemistry |
| Experience | Fresher to 3–5+ Years depending on role |