Stallion Hiring Production, QC, QA, ADL, PPMC

- Company Overview
- Job Role & Responsibilities
- Production – Sr. Officer / Executive
- Quality Control (QC) – Officer / Sr. Officer
- Quality Control – Microbiology (QC Micro)
- Quality Assurance (QA)
- Quality Assurance – Officer
- Analytical Development Laboratory (ADL)
- Production Planning & Material Control (PPMC)
- Eligibility / Qualifications
- Relevant Courses
- Location & Salary
- Application Process
- FAQs
- Summary Table
M.Pharm B.Pharm QC QA ADL Jobs | Stallion Labs
Stallion Laboratories hiring Production, QC, QA, ADL, PPMC professionals. Multiple vacancies for B.Pharm, M.Pharm, MSc with 1–8 years experience.
Stallion Laboratories is expanding its pharmaceutical manufacturing and quality operations and is inviting experienced professionals to join its regulated facility. This hiring drive covers key functions across Production, Quality Control, Quality Assurance, Analytical Development Laboratory (ADL), and Production Planning & Material Control (PPMC), offering stable growth, regulatory exposure, and long-term career progression in a GMP-compliant environment.
Company Overview
Stallion Laboratories is a quality-driven pharmaceutical organization engaged in manufacturing and analytical operations aligned with global regulatory standards. The company focuses on robust process control, strong quality systems, and continuous improvement across production, quality, and analytical functions. Professionals joining Stallion gain hands-on exposure to GMP, data integrity, and cross-functional collaboration essential for regulated pharmaceutical manufacturing.
Job Role & Responsibilities
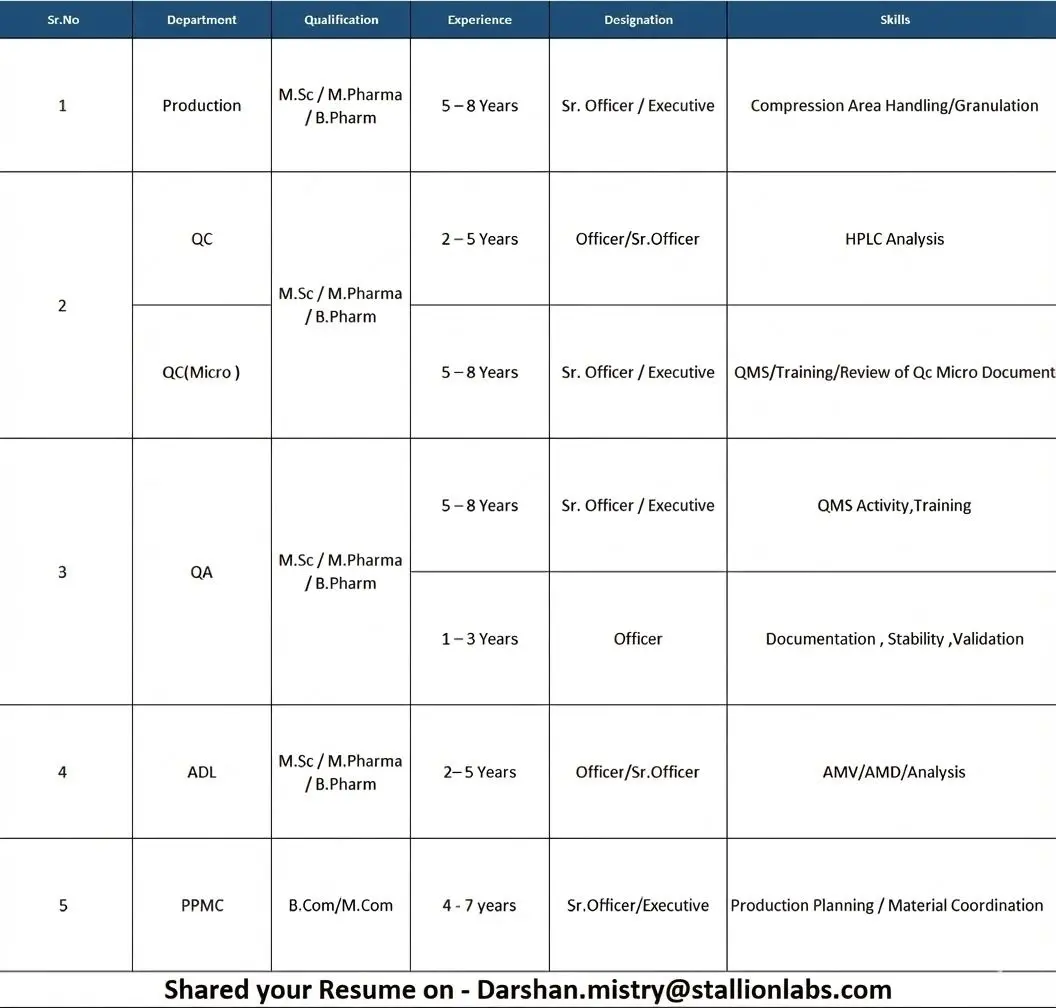
Production – Sr. Officer / Executive
Qualification: M.Sc, M.Pharm, B.Pharm
Experience: 5–8 years
Key Responsibilities:
- Handling compression and granulation operations
- Executing batch manufacturing activities as per approved BMRs
- Ensuring adherence to cGMP, SOPs, and safety guidelines
- Coordinating with QA and QC during in-process controls
- Maintaining production documentation and shift records
Quality Control (QC) – Officer / Sr. Officer
Qualification: M.Sc, M.Pharm, B.Pharm
Experience: 2–5 years
Key Responsibilities:
- Performing HPLC analysis for raw materials, in-process, and finished products
- Reviewing analytical data and ensuring data integrity compliance
- Calibration and maintenance of analytical instruments
- Supporting stability studies and method validation activities
Quality Control – Microbiology (QC Micro)
Qualification: M.Sc, M.Pharm, B.Pharm
Experience: 5–8 years
Key Responsibilities:
- Microbiological testing and environmental monitoring
- QMS activities related to QC Microbiology
- Review of microbiology documents, SOPs, and training records
- Ensuring compliance with GMP and regulatory microbiology standards
Quality Assurance (QA)
Qualification: M.Sc, M.Pharm, B.Pharm
Experience: 5–8 years
Key Responsibilities:
- Managing QMS activities and GMP documentation
- Conducting training programs and maintaining training records
- Supporting internal and external audits
- Ensuring compliance with regulatory and quality requirements
Quality Assurance – Officer
Qualification: M.Sc, M.Pharm, B.Pharm
Experience: 1–3 years
Key Responsibilities:
- Handling documentation, stability coordination, and validation activities
- Supporting deviation, change control, and CAPA documentation
- Assisting in batch record review and product release activities
Analytical Development Laboratory (ADL)
Qualification: M.Sc, M.Pharm, B.Pharm
Experience: 2–5 years
Key Responsibilities:
- Analytical method development and validation (AMD/AMV)
- Performing routine and development analysis
- Supporting method transfer and troubleshooting
- Documentation as per GLP and GMP guidelines
Production Planning & Material Control (PPMC)
Qualification: B.Com, M.Com
Experience: 4–7 years
Key Responsibilities:
- Production planning and scheduling
- Material coordination and inventory control
- Coordination with production, stores, and procurement teams
- Ensuring uninterrupted production flow through effective planning
Eligibility / Qualifications
- Accepted qualifications: B.Pharm, M.Pharm, M.Sc, B.Com, M.Com
- Relevant pharmaceutical manufacturing experience is mandatory
- Strong knowledge of GMP, documentation practices, and regulatory compliance
Relevant Courses
B.Pharm, M.Pharm, M.Sc Chemistry, M.Sc Pharmaceutical Sciences, B.Com, M.Com
Location & Salary
Job Location: As per company operations
Employment Type: Full-time
Salary will be offered based on experience, role complexity, and interview performance. There is no fixed salary cap for the right candidate.
Application Process
Interested candidates can share their updated resume via email:
Shortlisted candidates will be contacted for further interview steps.
FAQs
1. Is pharmaceutical experience mandatory?
Yes, relevant pharma industry experience is required for all roles.
2. Are analytical development roles available?
Yes, ADL positions are open for candidates with method development and validation experience.
3. Which departments are hiring?
Production, QC, QC Microbiology, QA, ADL, and PPMC.
4. What instruments should QC candidates be familiar with?
HPLC and related analytical instrumentation.
5. How do I apply?
Send your updated CV to Darshan.mistry@stallionlabs.com.
Summary Table
| Company | Stallion Laboratories |
|---|---|
| Vacancies | Production, QC, QC Micro, QA, ADL, PPMC |
| Required Education | B.Pharm, M.Pharm, M.Sc, B.Com, M.Com |
| Experience | 1–8 years depending on role |
You must sign in to apply for this position.


