Stivaph Hiring B.Pharm, M.Pharm, MBA, CA

- Company Overview
- Job Role & Responsibilities
- Production Department (Solid, Liquid & Ointment)
- Quality Assurance (QA)
- Quality Control (QC)
- Quality Control – Microbiology
- Regulatory Affairs
- Purchase Department
- Business Development
- Engineering Department
- Warehouse
- Human Resources & Administration
- Chartered Accountant (CA)
- Eligibility / Qualifications
- Location & Work Details
- Application Process
- Why Join Stivaph Healthcare?
- Frequently Asked Questions (FAQs)
- 1. Is pharma experience mandatory?
- 2. Can freshers apply?
- 3. Are multiple senior-level roles available?
- 4. How to apply?
- 5. Is this a plant-based role?
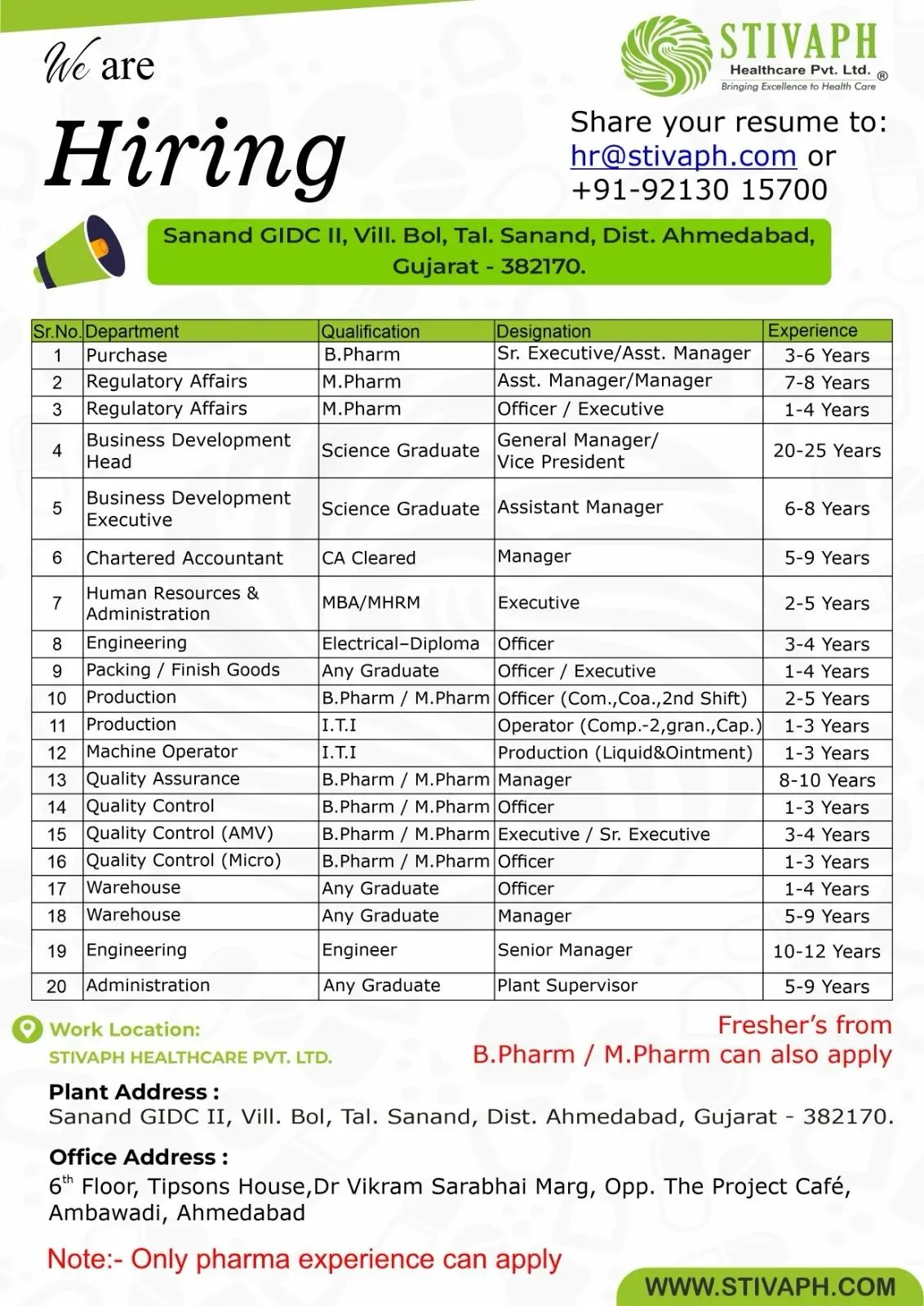
- Job Summary Table
B.Pharm, M.Pharm 20+ Vacancies Ahmedabad
B.Pharm, M.Pharm, MBA, CA hiring for 20+ pharma vacancies in Ahmedabad at Stivaph Healthcare Pvt. Ltd.
Stivaph Healthcare Pvt. Ltd. is expanding its pharmaceutical operations and inviting applications from experienced pharma professionals across multiple departments including Production, Quality Assurance, Quality Control, Regulatory Affairs, Engineering, Warehouse, Purchase, Business Development, HR, and Administration. This large-scale hiring drive is focused strictly on candidates with pharmaceutical industry experience, particularly those with exposure to GMP-compliant manufacturing environments.
If you are searching for pharma jobs in Ahmedabad, regulatory affairs careers in Gujarat, production manager roles in formulation plants, or quality control officer openings in a growing pharmaceutical company, this opportunity deserves attention.
Company Overview
Stivaph Healthcare Pvt. Ltd. operates from Sanand GIDC II, Ahmedabad, one of Gujarat’s rapidly developing pharmaceutical manufacturing zones. The company focuses on formulation development and commercial manufacturing, including liquid and ointment dosage forms.
With a structured plant setup and corporate office in Ahmedabad, Stivaph follows GMP guidelines and regulated documentation systems. The organization emphasizes quality compliance, validated processes, and cross-functional coordination across production, QA, QC, warehouse, and regulatory departments.
As the pharmaceutical sector continues to expand in regulated and semi-regulated markets, companies like Stivaph play a direct role in strengthening India’s formulation manufacturing capacity. Professionals joining the company gain exposure to pharmaceutical compliance systems, regulatory documentation, validation processes, and supply chain management — all critical for long-term career growth in life sciences.
Job Role & Responsibilities
Stivaph Healthcare is hiring across the following departments:
Production Department (Solid, Liquid & Ointment)
Designations: Operator, Officer, Executive, Manager
Experience Range: 1–10 Years
Key Responsibilities:
- Handling compression, coating, granulation, and capsule filling operations
- Managing liquid and ointment production lines
- Ensuring batch manufacturing record (BMR) compliance
- Supervising machine operators and shift activities
- Maintaining cGMP documentation and line clearance procedures
- Coordinating with QA for in-process quality checks
Machine Operators (ITI qualified) must have exposure to compression, granulation, and capsule filling equipment.
Quality Assurance (QA)
Designations: Executive, Sr. Executive, Manager
Experience: 3–10 Years
Key Responsibilities:
- Managing Quality Management Systems (QMS)
- Handling deviations, CAPA, change control
- Conducting internal audits and compliance reviews
- Batch record review and release documentation
- Supporting regulatory inspections and audit preparedness
QA professionals contribute directly to regulatory compliance and pharmaceutical quality systems integrity.
Quality Control (QC)
Designations: Officer, Executive, Sr. Executive
Experience: 1–4 Years
Key Responsibilities:
- Raw material and finished product testing
- Analytical Method Validation (AMV)
- Handling HPLC, UV, and other analytical instruments
- Stability studies and documentation
- OOS/OOT investigations
Quality Control – Microbiology
Designation: Officer
Experience: 1–3 Years
Responsibilities:
- Environmental Monitoring (EM)
- Microbial testing and culture handling
- Water system monitoring
- Sterility and contamination control documentation
Regulatory Affairs
Designation: Officer / Executive / Assistant Manager / Manager
Qualification: M.Pharm
Experience: 3–8 Years
Responsibilities:
- Dossier preparation (CTD/eCTD)
- Product registration documentation
- Regulatory submission coordination
- Variation filing and lifecycle management
Regulatory Affairs remains one of the highest growth and high CPC domains within the pharmaceutical sector.
Purchase Department
Designation: Sr. Executive / Assistant Manager
Qualification: B.Pharm
Experience: 3–6 Years
Responsibilities:
- Vendor management
- Raw material procurement
- Cost negotiation and compliance documentation
Business Development
Designation: Executive / Head / General Manager / Vice President
Qualification: Science Graduate / MBA
Experience: 2–25 Years (Based on role)
Responsibilities:
- Market expansion strategy
- Contract manufacturing acquisition
- Client relationship management
- Revenue growth planning
Engineering Department
Designation: Engineer / Plant Supervisor / Senior Manager
Qualification: Electrical Diploma / Engineering Degree
Experience: 3–12 Years
Responsibilities:
- Preventive maintenance
- Utility and HVAC management
- Equipment qualification support
- Plant supervision and infrastructure reliability
Warehouse
Designation: Officer / Manager
Qualification: Any Graduate
Experience: 1–9 Years
Responsibilities:
- Inventory control
- Material inward and outward documentation
- GMP storage compliance
- Coordination with production and QA
Human Resources & Administration
Qualification: MBA / MHRM / Any Graduate
Experience: 2–5 Years
Responsibilities:
- Recruitment coordination
- Payroll and employee documentation
- Compliance with labor regulations
- Administrative operations
Chartered Accountant (CA)
Qualification: CA Cleared
Designation: Manager
Experience: 5–9 Years
Responsibilities:
- Financial compliance
- Audit handling
- Budget planning and cost control
Eligibility / Qualifications
Relevant educational backgrounds include:
B.Pharm, M.Pharm, B.Sc., M.Sc., MBA, MHRM, Chartered Accountant (CA), ITI, Diploma (Electrical), Engineering Degree, Science Graduate, Any Graduate (with pharma experience).
Relevant courses include: Pharmaceutics, Pharmaceutical Technology, Industrial Pharmacy, Regulatory Affairs, Analytical Chemistry, Microbiology, Biotechnology, Life Sciences, Finance, Human Resource Management, Mechanical Engineering, Electrical Engineering.
Important Note: Only candidates with pharmaceutical industry experience are eligible to apply.
Freshers from B.Pharm and M.Pharm backgrounds may apply for selected entry-level roles.
Location & Work Details
Plant Address:
Sanand GIDC II, Village Bol, Taluka Sanand, District Ahmedabad, Gujarat – 382170
Corporate Office:
6th Floor, Tipsons House, Dr. Vikram Sarabhai Marg, Opp. The Project Café, Ambawadi, Ahmedabad
Stivaph’s location in Sanand GIDC provides proximity to major pharma clusters and logistics networks, offering strong career stability in Gujarat’s pharmaceutical manufacturing ecosystem.
Salary will be aligned with experience, designation level, and industry standards.
Application Process
Interested candidates can share their updated resume at:
hr@stivaph.com
Contact: +91-92130 15700
Website: www.stivaph.com
Ensure your resume clearly mentions:
- Department applied for
- Total pharma experience
- Current CTC and expected CTC
- Notice period
Shortlisted candidates will be contacted for further technical and HR discussions.
Why Join Stivaph Healthcare?
- Multi-department career growth opportunities
- Exposure to regulated pharmaceutical manufacturing
- Structured GMP and quality systems
- Leadership openings up to Vice President level
- Strong presence in Gujarat pharma manufacturing hub
Professionals seeking pharmaceutical production jobs in Ahmedabad, QA manager openings, QC analytical method validation careers, regulatory affairs manager roles, or pharma business development leadership positions should evaluate this opportunity seriously.
Frequently Asked Questions (FAQs)
1. Is pharma experience mandatory?
Yes. Only candidates with pharmaceutical industry experience are eligible.
2. Can freshers apply?
Freshers from B.Pharm and M.Pharm can apply for select entry-level roles.
3. Are multiple senior-level roles available?
Yes. Positions range from Operator and Officer to Senior Manager and Vice President levels.
4. How to apply?
Send your resume to hr@stivaph.com or contact +91-92130 15700.
5. Is this a plant-based role?
Yes. Most roles are based at the Sanand GIDC II manufacturing plant.
Job Summary Table
| Company | Stivaph Healthcare Pvt. Ltd. |
|---|---|
| Vacancies | Production Operator, QA Executive, QC Officer (AMV), QC Micro Officer, Regulatory Affairs Manager, Purchase Executive, Business Development Executive/VP, Engineering Supervisor, Warehouse Officer/Manager, HR Executive, Chartered Accountant |
| Required Education | B.Pharm, M.Pharm, B.Sc., M.Sc., MBA, MHRM, CA, ITI, Diploma, Engineering Degree, Any Graduate (Pharma Experience) |
| Experience | 1–25 Years (Role Dependent) |
To apply for this job email your details to hr@stivaph.com


