Sun Hiring MS&T Injectable, MES Engineer, Regulatory Affairs

- Company Overview
- Job Role & Responsibilities
- Eligibility / Qualifications
- Relevant Courses
- Location & Salary
- Application Process
- FAQs
- Summary Table
M.Pharm Vacancies MS&T RA MES | Sun Pharma Halol
Sun Pharma hiring MS&T Injectable, MES Engineer, Regulatory Affairs professionals. Multiple M.Pharm vacancies in Halol and Vadodara.
Sun Pharmaceutical Industries Ltd. is hiring experienced M.Pharm professionals for critical roles in MS&T Injectables, MES Engineering, and Regulatory Affairs (US Group). These opportunities are based at Sun Pharma’s advanced manufacturing and regulatory hubs in Halol and Vadodara and are ideal for professionals seeking leadership exposure in regulated injectable manufacturing and global regulatory submissions.
Company Overview
Sun Pharmaceutical Industries Ltd. is India’s largest pharmaceutical company and a globally trusted name in specialty and generic medicines. With a strong presence across regulated markets including the US, EU, and Canada, Sun Pharma operates world-class manufacturing facilities and R&D centers that comply with USFDA, EMA, and other global regulatory standards. The company is known for its focus on complex dosage forms, injectables, and high-quality regulatory science.
Job Role & Responsibilities
Sun Pharma is recruiting for the following high-impact positions:
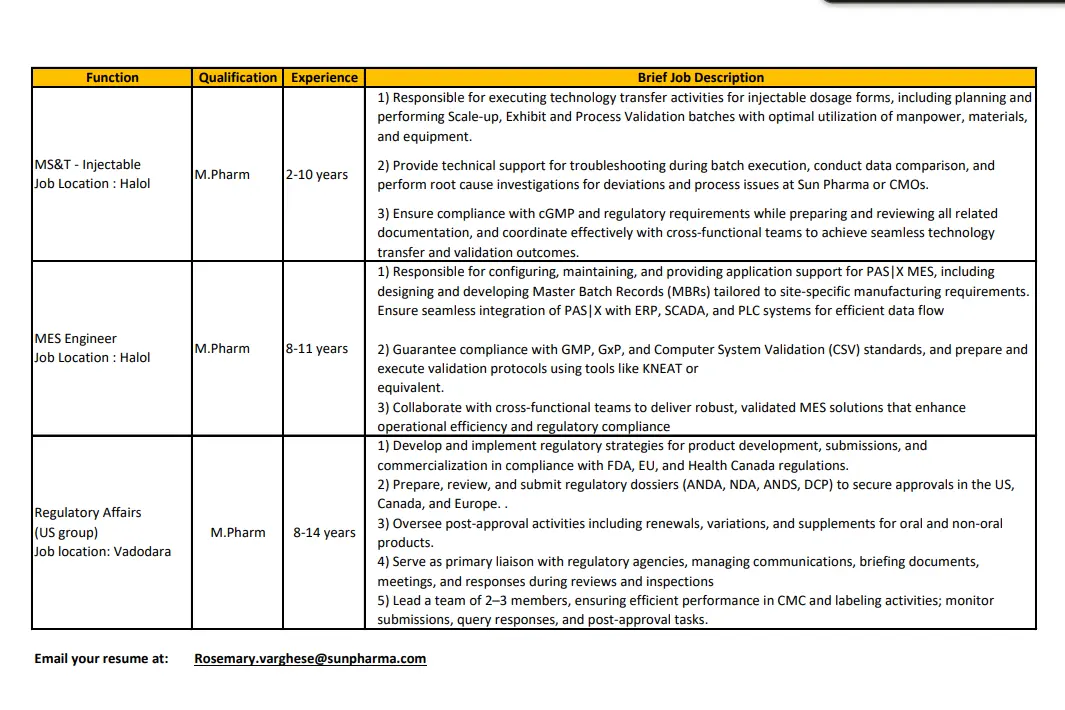
MS&T – Injectables (Manufacturing Science & Technology)
Job Location: Halol, Gujarat
Qualification: M.Pharm
Experience: 2–10 years
Key Responsibilities:
- Execution of technology transfer activities for injectable dosage forms
- Planning and execution of scale-up, exhibit, and process validation batches
- Troubleshooting during batch execution and investigation of deviations
- Data comparison, root cause analysis, and continuous process improvement
- Ensuring compliance with cGMP and regulatory requirements
- Preparation and review of MS&T and validation documentation
- Coordination with cross-functional teams including QA, QC, Production, and R&D
MES Engineer – PAS-X / PASIX
Job Location: Halol, Gujarat
Qualification: M.Pharm
Experience: 8–11 years
Key Responsibilities:
- Configuration, maintenance, and application support for PASIX MES systems
- Design and development of Master Batch Records (MBRs) aligned with site requirements
- Integration of MES with ERP, SCADA, and PLC systems
- Compliance with GMP, GxP, and Computer System Validation (CSV)
- Preparation and execution of validation protocols using KNEAT or equivalent tools
- Collaboration with IT, QA, Engineering, and Manufacturing teams
Regulatory Affairs – US Group
Job Location: Vadodara, Gujarat
Qualification: M.Pharm
Experience: 8–14 years
Key Responsibilities:
- Development and execution of regulatory strategies for US, EU, and Canada markets
- Preparation and submission of ANDA, NDA, ANDS, and DCP dossiers
- Management of post-approval activities including variations, supplements, and renewals
- Primary liaison with regulatory agencies during reviews and inspections
- Leadership of a small regulatory team handling CMC and labeling activities
- Oversight of submission timelines, agency queries, and regulatory commitments
Eligibility / Qualifications
- M.Pharm from a recognized institution
- Strong understanding of cGMP, validation, and regulatory guidelines
- Experience in injectables, MES systems, or global regulatory submissions as per role
Relevant Courses
M.Pharm Pharmaceutics, M.Pharm Pharmaceutical Technology, M.Pharm Industrial Pharmacy, M.Pharm Regulatory Affairs.
Location & Salary
Job Locations: Halol and Vadodara, Gujarat
Employment Type: Full-time
Salary will be offered based on experience, technical expertise, and interview performance. Senior roles carry leadership-level compensation packages.
Application Process
Interested candidates can apply by emailing their updated resume to:
Applicants are advised to clearly mention the position title in the subject line.
FAQs
1. Is injectable experience mandatory for MS&T roles?
Yes, hands-on injectable manufacturing or MS&T experience is required.
2. What MES platforms are preferred?
PAS-X / PASIX experience with ERP and automation integration is preferred.
3. Which regulatory markets does the RA role support?
US FDA, EU, and Health Canada.
4. Are leadership responsibilities involved?
Yes, the RA role includes team leadership responsibilities.
Summary Table
Company Sun Pharmaceutical Industries Ltd.
Vacancies MS&T Injectables, MES Engineer, Regulatory Affairs
Required Education M.Pharm
Experience 2–14 years depending on role
You must sign in to apply for this position.


