USV Pharma Walk-in QA, QC, and Production

- USV Pharma Hiring | QC, QA, Production Roles | Vadodara
- Company Overview
- Walk-In Interview Details
- Job Role & Responsibilities
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join USV Pvt. Ltd.?
- Frequently Asked Questions (FAQs)
- Summary Table
USV Pharma Hiring | QC, QA, Production Roles | Vadodara
Walk-in Drive at USV Pvt. Ltd. Vadodara on Nov 9, 2025 for QA, QC, and Production roles. B.Pharm, M.Pharm, M.Sc, ITI, Diploma candidates can apply.
Shape your pharma career with USV Private Limited, one of India’s most trusted and innovation-driven pharmaceutical companies. USV is conducting a Walk-In Interview Drive on Sunday, November 9, 2025, for experienced professionals across multiple departments including Quality Control (QC), Quality Assurance (QA), Production, and Packing at its Savli-Manjusar, Vadodara manufacturing facility. This opportunity is ideal for candidates passionate about contributing to world-class pharmaceutical production and quality excellence.
Company Overview
USV Private Limited is a leading Indian pharmaceutical organization with over 60 years of excellence in developing, manufacturing, and marketing high-quality pharmaceutical formulations. Known for its strong presence in chronic therapies, active pharmaceutical ingredients (APIs), and biosimilars, USV has built a reputation for scientific integrity and global compliance.
Its Savli-Manjusar, Vadodara facility is a state-of-the-art oral solid dosage (OSD) plant, equipped to meet stringent international standards including USFDA, MHRA, and WHO-GMP. The company’s commitment to research-driven growth and sustainable innovation continues to empower healthcare professionals and improve patient outcomes worldwide.
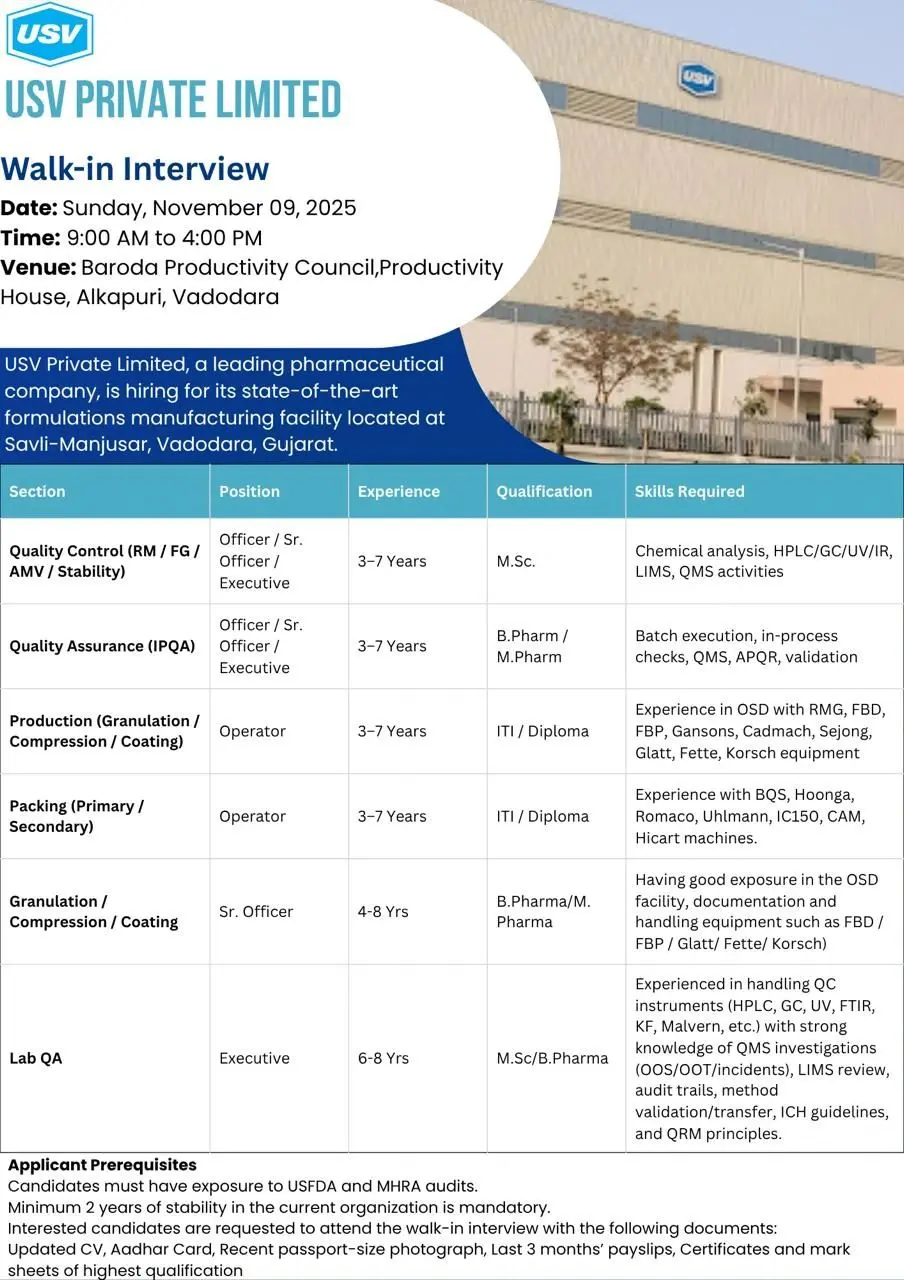
Walk-In Interview Details
Date: Sunday, November 9, 2025
Time: 9:00 AM – 4:00 PM
Venue: Baroda Productivity Council, Productivity House, Alkapuri, Vadodara
Work Location: USV Pvt. Ltd., Savli-Manjusar, Vadodara, Gujarat
Note: Candidates must have prior experience in regulated pharmaceutical environments.
Job Role & Responsibilities
USV is hiring experienced professionals across multiple departments:
1. Quality Control (QC – RM/FG/AMV/Stability)
- Position: Officer / Sr. Officer / Executive
- Qualification: M.Sc (Chemistry / Analytical Science)
- Experience: 3–7 Years
- Key Skills:
- Hands-on experience with HPLC, GC, UV, IR, and LIMS systems.
- Knowledge of QMS activities including CAPA, OOS, and OOT.
- Strong analytical and documentation skills.
- Experience in chemical and instrumental analysis of raw materials and finished goods.
2. Quality Assurance (IPQA)
- Position: Officer / Sr. Officer / Executive
- Qualification: B.Pharm / M.Pharm
- Experience: 3–7 Years
- Key Skills:
- In-process checks during manufacturing and packing.
- Knowledge of QMS, APQR, Validation, and Batch Execution.
- Exposure to USFDA/MHRA audit environments preferred.
3. Production (Granulation / Compression / Coating)
- Position: Operator
- Qualification: ITI / Diploma
- Experience: 3–7 Years
- Key Skills:
- Operation of RMG, FBD, FBP, Gansons, Glatt, Fette, Korsch, and Sejong equipment.
- Exposure to oral solid dosage (OSD) manufacturing in regulated plants.
4. Packing (Primary / Secondary)
- Position: Operator
- Qualification: ITI / Diploma
- Experience: 3–7 Years
- Key Skills:
- Hands-on experience with BQS, Hoonga, Romaco, Uhlmann, IC150, CAM, and Hicart packing lines.
- Understanding of visual inspection, labeling, and track & trace systems.
5. Granulation / Compression / Coating – Sr. Officer
- Qualification: B.Pharm / M.Pharm
- Experience: 4–8 Years
- Key Skills:
- Strong knowledge of OSD manufacturing processes, documentation, and validation.
- Equipment handling: FBD, FBP, Glatt, Fette, Korsch.
6. Lab QA (Quality Assurance – Laboratory)
- Position: Executive
- Qualification: M.Sc / B.Pharm
- Experience: 6–8 Years
- Key Skills:
- Expertise with HPLC, GC, UV, FTIR, KF, Malvern.
- Strong exposure to QMS Investigations (OOS/OOT/Incidents).
- LIMS review, audit trails, method validation and transfer, ICH guidelines, and QRM principles.
Eligibility / Qualifications
Educational Requirements:
- M.Sc (Chemistry, Analytical Science)
- B.Pharm / M.Pharm
- ITI / Diploma (Mechanical, Electrical, Instrumentation)
Experience Range: 3–8 Years (Department-Specific)
Key Prerequisites:
- Must have experience in regulated pharmaceutical manufacturing (USFDA / MHRA / WHO-GMP).
- Minimum 2 years of stability in the current organization.
- Excellent communication, teamwork, and documentation skills.
Relevant Courses (comma-separated): B.Pharmacy, M.Pharmacy, M.Sc Chemistry, M.Sc Analytical Science, Diploma in Engineering, ITI Technician.
Location & Salary
Work Location: Savli-Manjusar, Vadodara, Gujarat.
Work Mode: On-site (Manufacturing Facility).
Salary: Competitive, as per industry standards.
Experience: 3–8 Years.
Application Process
Candidates meeting the eligibility criteria can attend the walk-in interview directly at the venue with the required documents.
Documents to Carry:
- Updated Resume
- Aadhaar Card
- Recent Passport-size Photograph
- Last 3 Months’ Pay Slips
- Educational Certificates and Mark Sheets
Unable to Attend?
Share your CV via email to HR contact (as per company instructions) or follow USV’s official career page for upcoming opportunities.
Why Join USV Pvt. Ltd.?
- Opportunity to work in a globally compliant OSD facility.
- Exposure to international regulatory audits (USFDA, MHRA, WHO).
- Learn under industry veterans with strong technical expertise.
- Stable and transparent work environment with structured growth.
- Competitive compensation with long-term career advancement potential.
Frequently Asked Questions (FAQs)
1. Who can attend the walk-in drive?
Professionals with 3–8 years of experience in QA, QC, Production, or Packing departments from regulated pharma facilities.
2. Is prior regulatory audit exposure mandatory?
Yes, candidates must have experience with USFDA and MHRA audits.
3. What qualifications are required?
B.Pharm, M.Pharm, M.Sc, ITI, or Diploma with relevant department experience.
4. Are freshers eligible?
No, this drive is for experienced professionals only.
5. What should candidates bring for the interview?
Updated resume, Aadhaar card, recent photo, pay slips, and educational documents.
Summary Table
| Category | Details |
|---|---|
| Company | USV Private Limited |
| Vacancies | QA, QC, Production, Packing, Lab QA |
| Required Education | B.Pharm, M.Pharm, M.Sc, ITI, Diploma |
| Experience | 3–8 Years |
| Location | Savli-Manjusar, Vadodara, Gujarat |
| Walk-In Date | 9th November 2025 |
| Time | 9:00 AM – 4:00 PM |