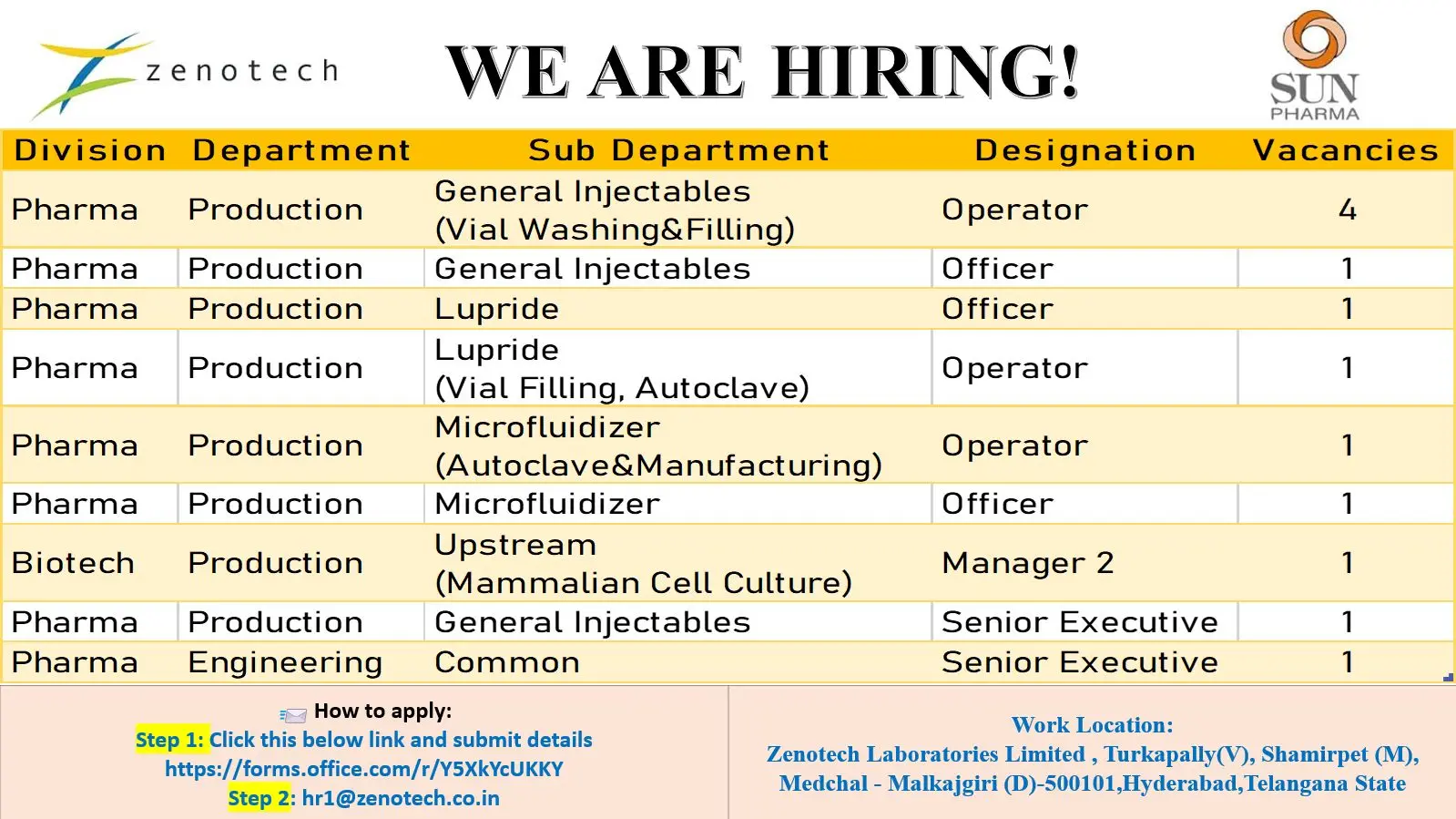
Zenotech Hiring Production & Engineering

- M.Pharm & B.Pharm Production Openings at Zenotech Hyderabad
- Company Overview
- Job Role & Responsibilities
- Production – General Injectables (Vial Washing & Filling)
- Production – General Injectables
- Production – Lupride
- Production – Lupride (Vial Filling, Autoclave)
- Production – Microfluidizer (Autoclave & Manufacturing)
- Production – Microfluidizer
- Biotech Production – Upstream (Mammalian Cell Culture)
- Production – General Injectables
- Engineering – Common
- Eligibility / Qualifications
- Location & Salary
- Application Process
- FAQs
- Summary Table
M.Pharm & B.Pharm Production Openings at Zenotech Hyderabad
Apply for multiple Production & Engineering roles at Zenotech Hyderabad. Openings for Operators, Officers, Executives, and Manager.
Zenotech Laboratories is expanding its production and biotechnology operations and hiring skilled professionals across injectables, Lupride, microfluidizer, and upstream cell culture. These roles suit candidates who want steady career growth in sterile manufacturing, biologics, and pharma engineering. The company is looking for individuals with strong technical hands-on experience and a commitment to quality-driven production.
Company Overview
Zenotech Laboratories Limited is a subsidiary of Sun Pharma and a trusted name in sterile injectables and biotechnology-driven products. The company operates advanced manufacturing units with a focus on general injectables, Lupride formulations, mammalian cell culture, and microfluidizer-assisted production. Zenotech follows global quality standards, modern compliance frameworks, and regulatory‑aligned production systems, making it a strong employer for candidates seeking long-term stability in pharma and biotech manufacturing.
Job Role & Responsibilities
The company has multiple openings across Production and Engineering. Each role requires attention to detail, adherence to cGMP, and the ability to handle sterile-area operations.
Production – General Injectables (Vial Washing & Filling)
Operator – 4 Vacancies
- Handle washing and depyrogenation of vials.
- Support sterile vial filling operations.
- Maintain production area hygiene and documentation.
- Operate filling lines and troubleshoot minor issues.
Production – General Injectables
Officer – 1 Vacancy
- Oversee manufacturing processes and batch activities.
- Review production documentation and ensure compliance.
- Support qualification and validation requirements.
Production – Lupride
Officer – 1 Vacancy
- Manage Lupride formulation steps and in‑process checks.
- Coordinate with QA and engineering for batch compliance.
Production – Lupride (Vial Filling, Autoclave)
Operator – 1 Vacancy
- Manage vial filling equipment for Lupride.
- Operate sterilization cycles and handle autoclave activities.
- Support line clearance and documentation.
Production – Microfluidizer (Autoclave & Manufacturing)
Operator – 1 Vacancy
- Run microfluidizer equipment for manufacturing sterile batches.
- Perform autoclave operations.
- Maintain logs and support supervision teams.
Production – Microfluidizer
Officer – 1 Vacancy
- Oversee microfluidization activities and ensure batch consistency.
- Manage equipment readiness, calibration, and documentation.
Biotech Production – Upstream (Mammalian Cell Culture)
Manager / Senior Manager – 2 Vacancies
- Handle upstream mammalian cell culture operations.
- Oversee fermenters, bioreactors, inoculum preparation.
- Drive compliance, batch execution, and deviation handling.
- Mentor teams and coordinate with cross-functional departments.
Production – General Injectables
Senior Executive – 1 Vacancy
- Supervise daily sterile operations.
- Review BMRs/BPRs and ensure timely batch execution.
- Support quality and regulatory audits.
Engineering – Common
Senior Executive – 1 Vacancy
- Oversee utility and equipment maintenance.
- Support production teams with timely engineering interventions.
- Document breakdowns and preventive maintenance.
Eligibility / Qualifications
- B.Pharmacy, M.Pharmacy, B.Sc, M.Sc, Biotechnology, Microbiology, Biochemistry.
- For Operators: ITI, Diploma candidates with sterile manufacturing exposure.
- Relevant experience in injectables, upstream biotechnology, autoclave operation, microfluidizer systems.
- Strong understanding of cGMP, aseptic practices, and documentation.
Location & Salary
Work Location:
Zenotech Laboratories Limited, Turkapally (V), Shamirpet (M), Medchal‑Malkajgiri (D), 500101, Hyderabad, Telangana.
Salary is based on qualification, experience, and interview performance. Biotech upstream and sterile production roles typically offer competitive compensation aligned with industry standards.
Application Process
Step 1: Submit your details using the online form:
Click to Apply
Step 2: Email your updated resume to:
hr1@zenotech.co.in
FAQs
1. Who can apply for Operator roles?
Candidates with ITI, Diploma, or B.Sc backgrounds and experience in vial washing, filling, autoclave, or sterile manufacturing.
2. Are freshers eligible?
Most roles require hands-on experience. Freshers may apply only if they have industrial training exposure.
3. What is the selection process?
Shortlisted candidates will be contacted for technical interviews and an on‑site assessment.
4. Is biotech experience mandatory for upstream roles?
Yes. Mammalian cell culture and bioreactor experience is required for Manager‑level upstream biotechnology positions.
5. How soon should candidates apply?
Applications are reviewed on a rolling basis. Early submission increases the chance of fast shortlisting.
Summary Table
| Company | Zenotech Laboratories Limited |
|---|---|
| Vacancies | Multiple openings across Production, Biotech, and Engineering |
| Required Education | B.Pharm, M.Pharm, B.Sc, M.Sc, Biotechnology, Microbiology, Biochemistry, ITI, Diploma |
| Experience | Relevant experience in injectables, sterile manufacturing, microfluidizer, upstream biotech, or engineering |
To apply for this job please visit forms.office.com.


