medifodil Hiring Regulatory Affairs, QA & BD Intern

- Company Overview
- Job Role & Responsibilities
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join Medifodil?
- FAQs
- Summary Table
M.Pharm, B.Pharm Vacancies | Medifodil Smart Pharma Solutions Careers 2025
Apply for Pharmacovigilance, Regulatory Affairs, QA & BD Intern roles at Medifodil Smart Pharma Solutions. Openings for B.Pharm/M.Pharm in Hyderabad.
Medifodil Smart Pharma Solutions, a trusted name in the pharmaceutical industry, is inviting talented and passionate professionals to join its dynamic team. Whether you are an experienced Pharmacovigilance Manager or a fresh graduate looking to begin your career in Business Development, Medifodil offers a diverse range of career opportunities designed to foster growth, innovation, and excellence in healthcare.
Company Overview
Medifodil Smart Pharma Solutions is a leading provider of integrated pharmaceutical services, specializing in Pharmacovigilance (PV), Regulatory Affairs, Quality Assurance, and Business Development. Known for its commitment to quality and regulatory compliance, Medifodil partners with global clients to deliver efficient, compliant, and reliable pharma solutions. The company is backed by a team of domain experts who ensure excellence across the drug development and post-marketing lifecycle.
With a strong emphasis on innovation, technology integration, and process excellence, Medifodil is building a workforce that contributes directly to patient safety and healthcare advancement.
Job Role & Responsibilities
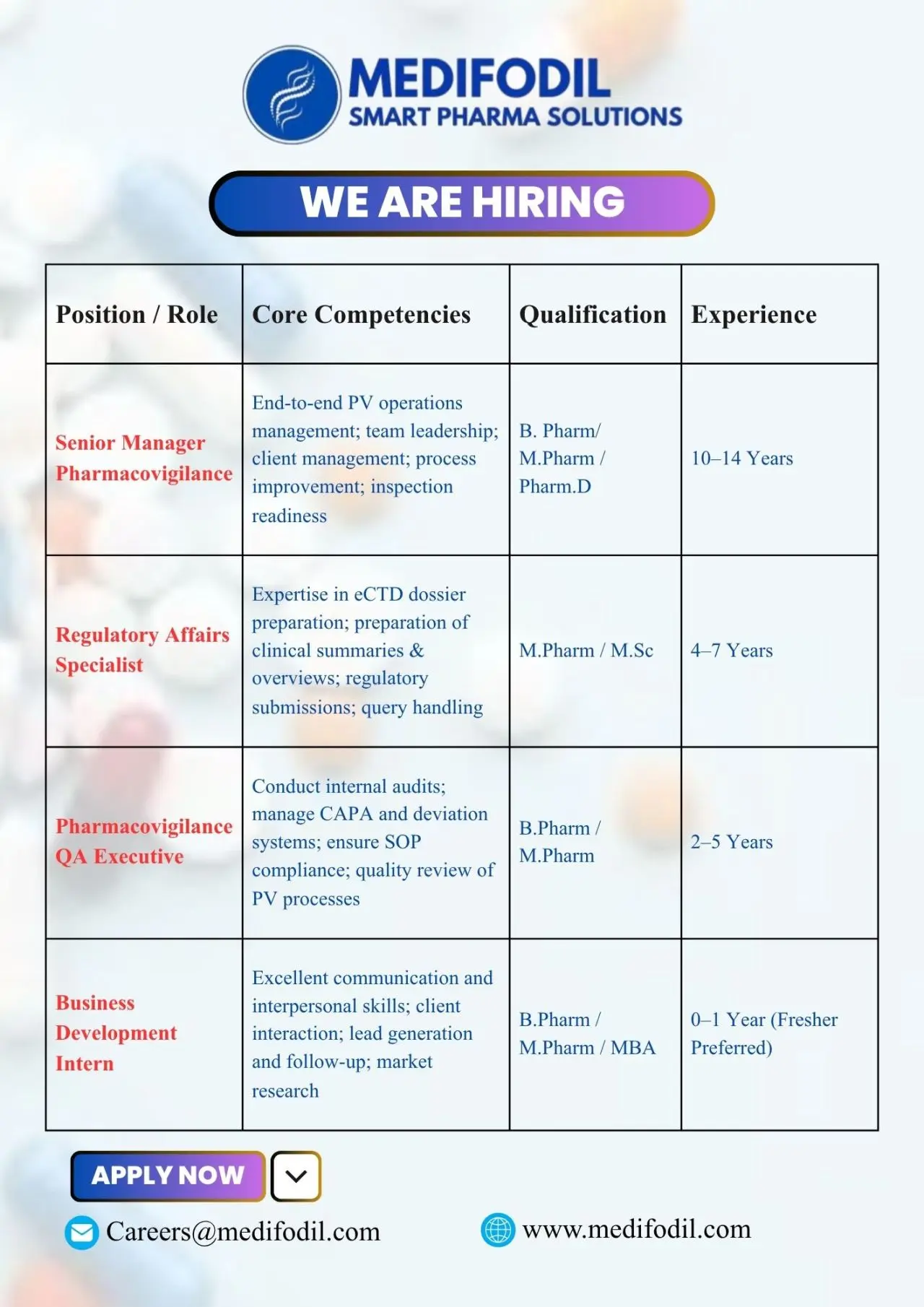
1. Senior Manager – Pharmacovigilance (PV)
Key Responsibilities:
- Oversee end-to-end pharmacovigilance operations and safety data management.
- Lead and mentor PV teams to ensure smooth process execution.
- Manage client communications and ensure timely project delivery.
- Ensure inspection readiness and maintain global compliance standards.
- Drive process improvement initiatives across the PV domain.
2. Regulatory Affairs Specialist
Key Responsibilities:
- Prepare, review, and submit eCTD dossiers for global regulatory submissions.
- Draft clinical summaries, overviews, and quality documents as per international guidelines.
- Handle regulatory queries efficiently and maintain documentation for audit readiness.
- Collaborate with cross-functional teams to ensure successful product registrations.
3. Pharmacovigilance QA Executive
Key Responsibilities:
- Conduct internal quality audits for pharmacovigilance processes.
- Manage CAPA (Corrective and Preventive Actions) and deviation systems.
- Ensure SOP adherence and process compliance across PV functions.
- Perform quality review of case processing and aggregate reports.
4. Business Development Intern
Key Responsibilities:
- Conduct market research and identify potential business opportunities.
- Generate leads, interact with clients, and support business proposals.
- Assist in client communication and follow-ups.
- Collaborate with senior BD professionals to enhance client acquisition strategies.
Eligibility / Qualifications
For Senior Manager – PV: B.Pharm, M.Pharm, or Pharm.D with 10-14 years of experience in pharmacovigilance operations and team leadership.
For Regulatory Affairs Specialist: M.Pharm or M.Sc with 4-7 years of hands-on experience in regulatory dossier preparation and submissions.
For Pharmacovigilance QA Executive: B.Pharm or M.Pharm with 2-5 years of experience in PV quality systems, CAPA, and internal audits.
For Business Development Intern: B.Pharm, M.Pharm, or MBA with 0-1 year of experience; freshers are encouraged to apply.
Relevant Courses: B.Pharm, M.Pharm, Pharm.D, M.Sc (Regulatory Affairs, Pharmacology, Clinical Research), MBA (Pharma Management, Healthcare).
Location & Salary
Location: Hyderabad, India
Salary: Competitive package based on experience and qualifications. Fresher roles include performance-based incentives and growth opportunities.
Application Process
Interested candidates can send their updated resumes to
careers@medifodil.com
Visit: www.medifodil.com
Apply before 31st October 2025 to secure your interview slot!
Why Join Medifodil?
- Work with a rapidly growing pharmaceutical solutions provider.
- Exposure to global pharmacovigilance and regulatory systems.
- Opportunity to collaborate with top-tier clients and experts.
- Competitive pay scale and growth-oriented career path.
- Contribute to improving patient safety and regulatory excellence worldwide.
FAQs
Q1. What is the minimum qualification required for Medifodil positions?
A. Minimum qualification is B.Pharm or M.Pharm, depending on the role.
Q2. Are freshers eligible to apply?
A. Yes, freshers can apply for the Business Development Intern role.
Q3. How can I apply for these openings?
A. Send your resume to careers@medifodil.com or visit www.medifodil.com for more details.
Q4. What is the interview process?
A. Shortlisted candidates will be contacted via email or phone for interviews.
Q5. Does Medifodil offer remote work options?
A. Some roles may offer hybrid or remote flexibility based on project needs.
Summary Table
| Company | Medifodil Smart Pharma Solutions |
| Vacancies | Senior Manager PV, Regulatory Affairs Specialist, PV QA Executive, BD Intern |
| Required Education | B.Pharm, M.Pharm, Pharm.D, M.Sc, MBA |
| Experience | 0–14 years (depending on role) |
To apply for this job email your details to sudheer45227@gmail.com


