Production Operator, Manufacturing Officer, QMS Executive, QA Officer, Validation Officer, IPQA Officer, QC Officer HPLC, Microbiologist

- Company Overview
- Job Role & Responsibilities
- Production Department – Injection (Dry Powder Injection)
- Production Department – Oral
- Quality Assurance (QA) Department
- Quality Control (QC) Department
- Microbiology Department
- Eligibility / Qualifications
- Required Education
- Experience Requirements
- Location & Salary
- Interview & Application Process
- Frequently Asked Questions (FAQs)
- Is this hiring open for ITI candidates?
- Are freshers eligible for these roles?
- Is this a walk-in interview?
- Is experience in injectable manufacturing mandatory?
- Where is the job location?
- Summary Table
Ceph Lifesciences hiring for Production, QA, QC, Microbiology roles in Baddi. Multiple vacancies for B.Pharm, M.Pharm, ITI.
Ceph Lifesciences Pvt. Ltd. is conducting large-scale hiring for experienced and skilled professionals across Production, Quality Assurance, Quality Control, and Microbiology departments at its manufacturing facility in Baddi, Himachal Pradesh. This hiring drive is ideal for candidates with pharma manufacturing, quality, and laboratory experience who are looking for stable roles in a regulated pharmaceutical environment.
Company Overview
Ceph Lifesciences Pvt. Ltd. is a pharmaceutical manufacturing company with a strong presence in injectable and oral dosage forms. The organization focuses on compliance-driven manufacturing, quality systems, and continuous improvement aligned with national and international regulatory standards. Its Baddi facility is equipped with modern infrastructure supporting sterile manufacturing, quality control laboratories, and microbiology testing units.
Ceph Lifesciences emphasizes operational excellence, data integrity, and patient safety while offering long-term career growth for professionals in production, QA, QC, and microbiology functions.
Job Role & Responsibilities
Ceph Lifesciences is hiring for multiple roles across departments. Responsibilities vary by function but are aligned with GMP compliance, quality systems, and pharmaceutical manufacturing standards.
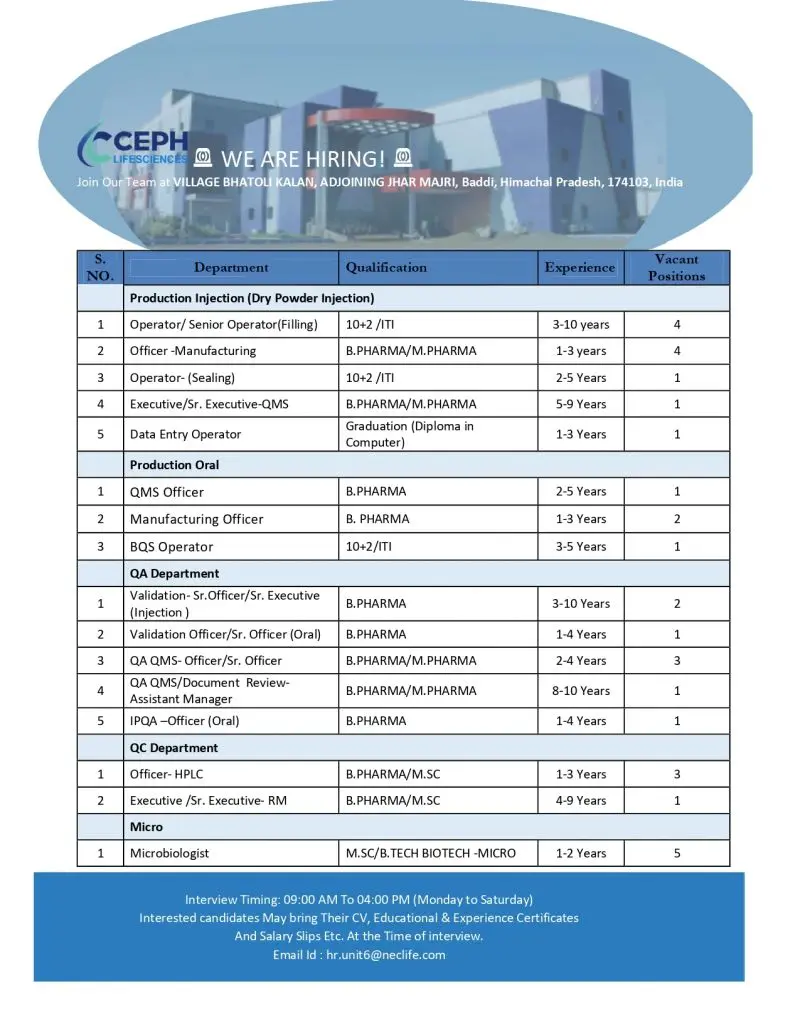
Production Department – Injection (Dry Powder Injection)
- Operator / Senior Operator (Filling)
- Officer – Manufacturing
- Operator – Sealing
- Executive / Senior Executive – QMS
- Data Entry Operator
Key responsibilities include operation of filling and sealing machines, batch manufacturing activities, documentation as per GMP, data entry, and adherence to SOPs and safety practices.
Production Department – Oral
- QMS Officer
- Manufacturing Officer
- BQS Operator
Responsibilities include oral dosage manufacturing, in-process controls, documentation, deviation handling, and quality system compliance.
Quality Assurance (QA) Department
- Validation Officer / Senior Officer / Senior Executive (Injection)
- Validation Officer / Senior Officer (Oral)
- QA QMS Officer / Senior Officer
- QA QMS / Document Review – Assistant Manager
- IPQA Officer (Oral)
Roles involve validation activities, batch record review, QMS documentation, deviation and change control handling, and IPQA oversight.
Quality Control (QC) Department
- Officer – HPLC
- Executive / Senior Executive – Raw Material
Responsibilities include chemical analysis, HPLC operation, raw material testing, documentation, and compliance with laboratory SOPs.
Microbiology Department
- Microbiologist
The role includes environmental monitoring, sterility testing, microbiological analysis, and compliance with aseptic and GMP requirements.
Eligibility / Qualifications
Required Education
- 10+2, ITI
- B.Pharmacy
- M.Pharmacy
- B.Sc
- M.Sc
- B.Tech Biotechnology
- Diploma in Computer Applications
Experience Requirements
Experience requirements range from 1 to 10 years depending on the role and department:
- Production Injection: 1 to 10 years
- Production Oral: 1 to 5 years
- QA Department: 1 to 10 years
- QC Department: 1 to 9 years
- Microbiology: 1 to 2 years
Location & Salary
Job Location: Baddi, Himachal Pradesh
Interview & Application Process
Interview Timing: 09:00 AM to 04:00 PM (Monday to Saturday)
Interested candidates should attend the interview with:
- Updated CV
- Educational certificates
- Experience certificates
- Latest salary slips
Candidates may also send their resumes via email:
Frequently Asked Questions (FAQs)
Is this hiring open for ITI candidates?
Yes. ITI and 10+2 candidates are eligible for selected production and BQS operator roles.
Are freshers eligible for these roles?
Most positions require prior experience. Freshers may be considered only for select entry-level roles based on interview performance.
Is this a walk-in interview?
Yes. Candidates can directly attend the interview during the mentioned timings.
Is experience in injectable manufacturing mandatory?
Injectable experience is mandatory for injection-related production and QA validation roles.
Where is the job location?
All positions are based at Ceph Lifesciences’ Baddi, Himachal Pradesh facility.
Summary Table
| Category | Details |
|---|---|
| Company | Ceph Lifesciences Pvt. Ltd. |
| Vacancies | Production Operator, Manufacturing Officer, QMS Executive, QA Officer, Validation Officer, IPQA Officer, QC Officer HPLC, Microbiologist |
| Required Education | 10+2, ITI, B.Pharm, M.Pharm, B.Sc, M.Sc, B.Tech Biotechnology, Diploma in Computer |
| Experience | 1 to 10 years depending on role |
To apply for this job email your details to hr.unit6@neclife.com


