USV Hiring QC, QA, IPQA, Validation & Engineering

- Company Overview
- Job Role & Responsibilities
- Department-Wise Openings:
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join USV Pvt Ltd?
- FAQs
- Job Summary Table
USV Pvt Ltd Hiring – QC, QA, IPQA, Validation, Engineering | Daman
USV Pvt Ltd Daman hiring for QC, QA, IPQA, Validation & Engineering. M.Sc/B.Pharm/B.E, 4–14 yrs exp. Apply via email.
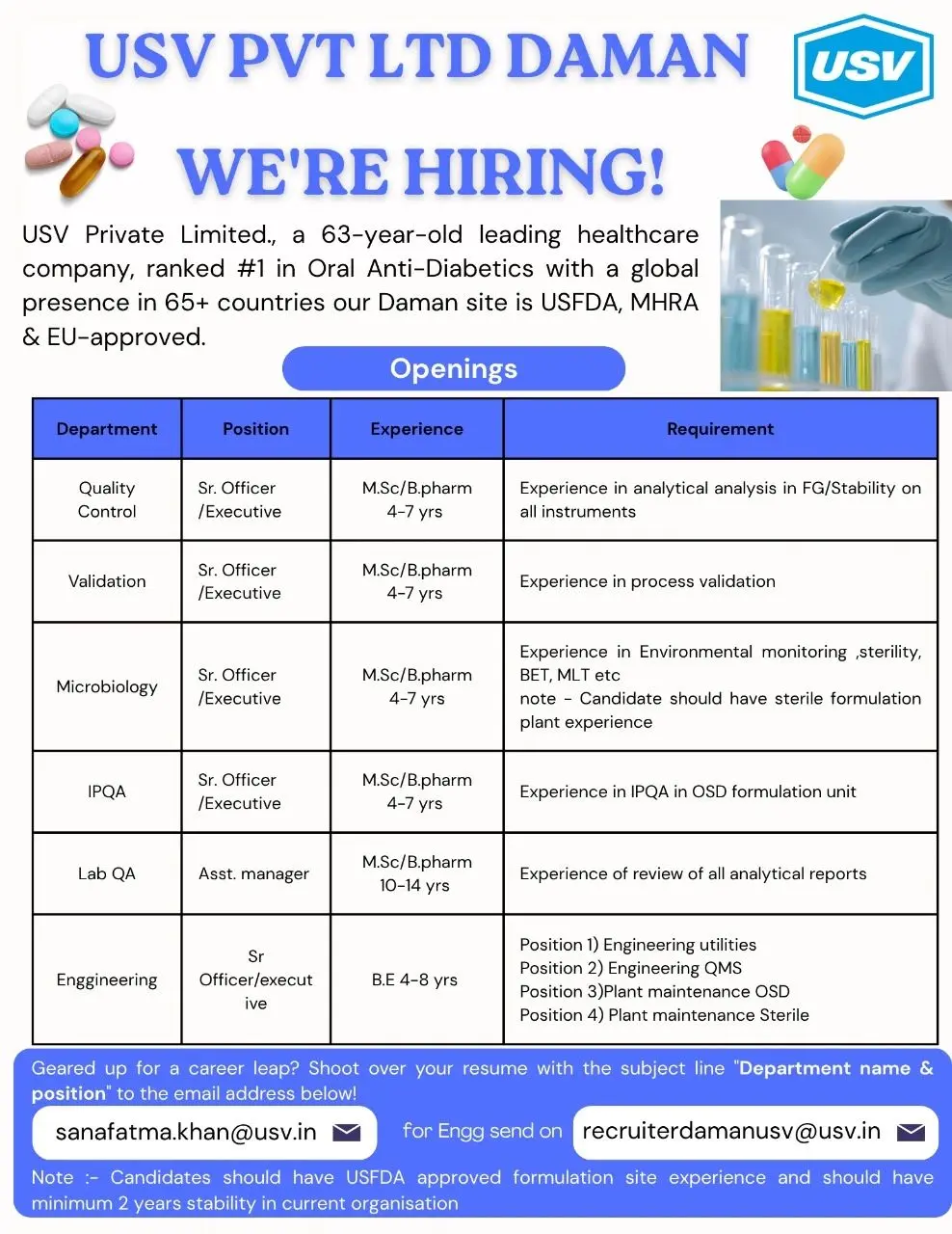
USV Private Limited, a 63-year-old trusted healthcare company with a strong global presence, is hiring experienced pharma professionals for multiple departments at its USFDA, MHRA, and EU-approved Daman facility. This opportunity is ideal for candidates looking to grow in a globally recognized pharmaceutical organization that values innovation, quality, and employee development.
Company Overview
USV Pvt Ltd is a leading pharmaceutical company headquartered in Mumbai, India. Established in 1961, USV is ranked #1 in Oral Anti-Diabetics and is a key player in the fields of cardiovascular, metabolic, and dermatological therapies. With operations in 65+ countries and manufacturing facilities approved by USFDA, EMA, and WHO-GMP, USV stands as a global leader in healthcare solutions.
The Daman site is one of USV’s most advanced manufacturing units, equipped with cutting-edge technology for the production of oral solid dosage (OSD) and sterile formulations. The facility adheres to global regulatory standards and is driven by a culture of continuous improvement, compliance, and operational excellence.
Job Role & Responsibilities
Department-Wise Openings:
1. Quality Control (QC)
Position: Sr. Officer / Executive
Qualification: M.Sc / B.Pharm
Experience: 4–7 Years
Requirement:
- Experience in analytical analysis of Finished Goods (FG) and Stability samples.
- Proficient in operating instruments such as HPLC, GC, UV, and Dissolution Apparatus.
- Experience in method validation, calibration, and data integrity practices.
2. Validation
Position: Sr. Officer / Executive
Qualification: M.Sc / B.Pharm
Experience: 4–7 Years
Requirement:
- Hands-on experience in process validation and equipment qualification.
- Familiarity with protocol preparation, execution, and report compilation.
- Understanding of cGMP and regulatory validation guidelines.
3. Microbiology
Position: Sr. Officer / Executive
Qualification: M.Sc / B.Pharm
Experience: 4–7 Years
Requirement:
- Expertise in environmental monitoring, sterility testing, BET, and MLT.
- Experience in sterile formulation facilities is mandatory.
- Knowledge of microbiological documentation, validation, and aseptic techniques.
4. In-Process Quality Assurance (IPQA)
Position: Sr. Officer / Executive
Qualification: M.Sc / B.Pharm
Experience: 4–7 Years
Requirement:
- Experience in IPQA operations in OSD formulation plants.
- Line clearance, in-process checks, and deviation handling.
- Good understanding of QMS, CAPA, and documentation review.
5. Lab QA
Position: Assistant Manager
Qualification: M.Sc / B.Pharm
Experience: 10–14 Years
Requirement:
- Review of analytical reports and instrument data.
- Experience in lab audits, OOS/OOT investigations, and QMS activities.
- Expertise in data integrity, documentation control, and compliance verification.
6. Engineering
Position: Sr. Officer / Executive
Qualification: B.E. (Mechanical / Electrical / Instrumentation preferred)
Experience: 4–8 Years
Open Positions:
- Engineering – Utilities
- Engineering – QMS
- Plant Maintenance – OSD
- Plant Maintenance – Sterile
Requirement:
- Strong understanding of preventive maintenance, HVAC, and equipment qualification.
- Familiarity with engineering documentation and compliance protocols.
- Exposure to regulatory audits in sterile and OSD environments.
Eligibility / Qualifications
Educational Qualifications: M.Sc, B.Pharm, or B.E. (Engineering) depending on the department.
Experience Range: 4–14 years depending on the role.
Industry: Pharmaceutical Formulations
Preferred Background: Candidates from USFDA-approved formulation plants with at least 2 years of stability in their current organization.
Relevant Courses: Industrial Pharmacy, Analytical Chemistry, Pharmaceutical Quality Control, Process Validation, Microbiology, Mechanical Engineering, Electrical Systems, Quality Management Systems.
Location & Salary
Location: Daman
Company: USV Pvt Ltd
Salary: Competitive, based on experience and qualifications
Employment Type: Full-Time, Permanent
Facility: USFDA, MHRA, and EU-approved site
USV offers an inclusive work environment with global exposure, training programs, and growth opportunities for ambitious professionals in pharma manufacturing.
Application Process
Interested and eligible candidates may send their updated CVs with the subject line as “Department Name & Position” to the email addresses below:
For All Departments (Except Engineering): sanafatma.khan@usv.in
For Engineering Positions: recruiterdamanusv@usv.in
Why Join USV Pvt Ltd?
- Work with one of India’s top-ranked pharmaceutical companies with a global footprint in 65+ countries.
- Opportunity to contribute to regulated manufacturing processes in USFDA-approved facilities.
- Exposure to high-quality production systems, compliance audits, and global standards.
- Strong focus on employee development, continuous learning, and internal growth.
- Competitive salary, professional stability, and performance-based incentives.
FAQs
1. What are the qualifications required?
Candidates with M.Sc, B.Pharm, or B.E depending on the department are eligible.
2. What is the experience range?
Applicants must have 4–14 years of experience in relevant pharma functions.
3. Is experience in USFDA-approved facilities mandatory?
Yes, candidates must have experience in a regulated USFDA-approved formulation site.
4. How do I apply?
Send your updated CV with your department and position in the subject line to the respective HR email ID listed above.
5. What departments are currently hiring?
Openings are available in QC, QA, IPQA, Validation, Microbiology, and Engineering.
6. What is the job location?
All positions are based at USV’s Daman site, a globally approved formulation facility.
Job Summary Table
| Category | Details |
|---|---|
| Company | USV Pvt Ltd |
| Vacancies | Multiple – QC, QA, Validation, Microbiology, IPQA, Engineering |
| Required Education | M.Sc / B.Pharm / B.E |
| Experience | 4–14 Years (Formulation Manufacturing) |
| Location | Daman |
| Email (QA/QC/IPQA) | sanafatma.khan@usv.in |
| Email (Engineering) | recruiterdamanusv@usv.in |
| Salary | Competitive, as per industry standards |
To apply for this job email your details to sanafatma.khan@usv.in


