Eris Hiring QC, QA, Manufacturing & Warehouse

- M.Pharm/B.Pharm QC & Manufacturing Roles — Eris Therapeutics
- Company Overview
- Job Role & Responsibilities
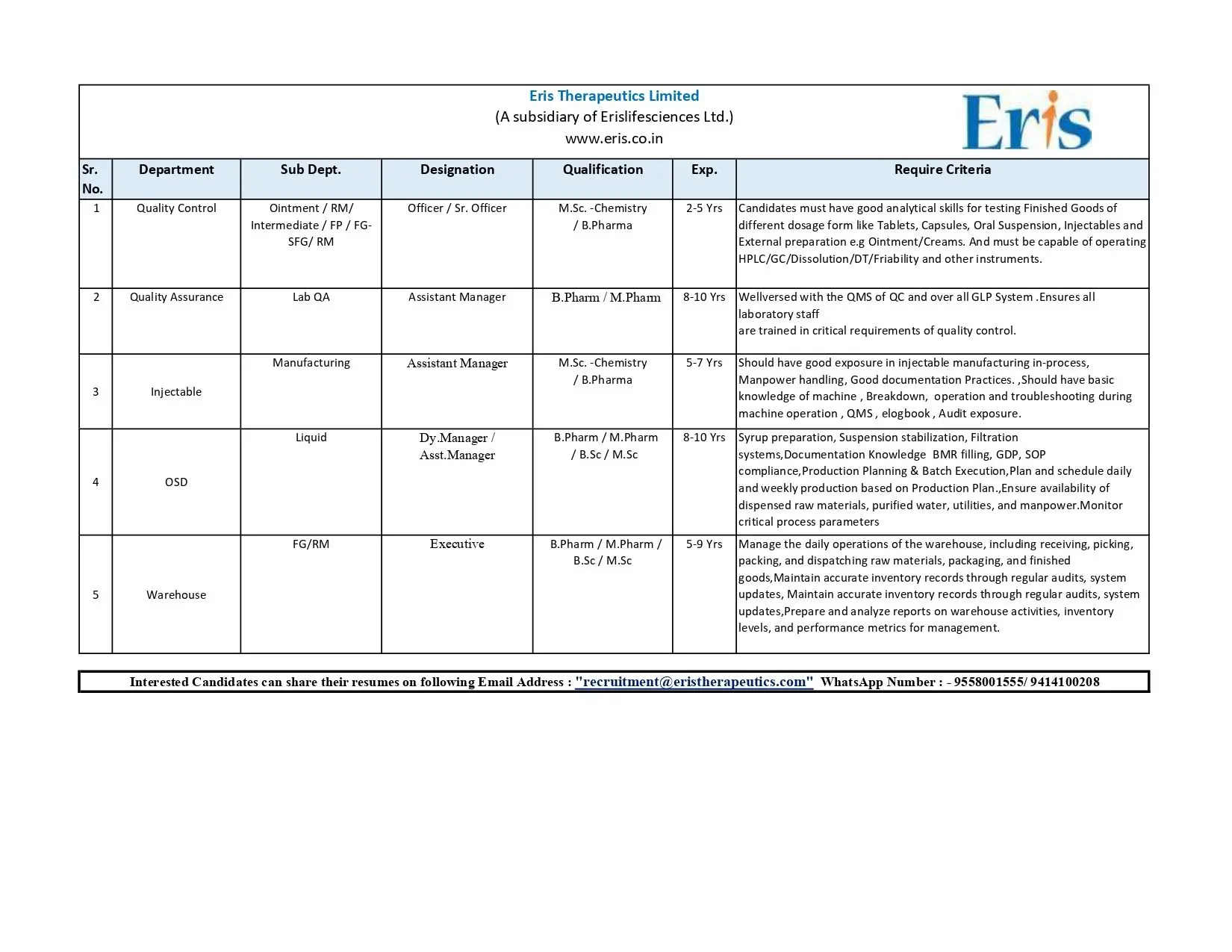
- Quality Control — Officer / Sr. Officer (Ointment / RM / Intermediate / FP / FG — OSD & External Preparations)
- Quality Assurance — Assistant Manager (Lab QA)
- Manufacturing — Assistant Manager (Injectable & OSD Operations)
- Injectable — Dy. Manager / Asst. Manager (Liquid Injectables)
- FG/RM Warehouse — Executive
- Eligibility / Qualifications
- Application Process
- FAQs
M.Pharm/B.Pharm QC & Manufacturing Roles — Eris Therapeutics
M.Sc/M.Pharm/B.Pharm openings in QC, QA, Manufacturing & Warehouse at Eris Therapeutics. Multiple vacancies across injectable & OSD. Apply now.
Eris Therapeutics Ltd (a subsidiary of Erislifesciences Ltd.) is recruiting experienced professionals across Quality Control, Quality Assurance, Manufacturing (Injectable & OSD) and Warehouse functions. If you are skilled in HPLC/GC operation, dissolution/friability testing, GMP documentation, injectable batch operations or warehouse inventory management — these roles at Eris offer a chance to work in regulated manufacturing with real operational responsibility and career growth.
Company Overview
Eris Therapeutics is part of the Erislifesciences group and operates with a clear focus on quality, regulatory compliance and scalable manufacturing. The company develops and manufactures a wide range of dosage forms — tablets, capsules, injectables, syrups and topical formulations — supplying both domestic and export markets. Eris invests in laboratory systems, QMS and training to maintain audit-readiness and product quality.
Joining Eris means working in a company that treats product quality as non-negotiable, invests in employee skill development and expects measurable accountability in technical roles.
Job Role & Responsibilities
Below is a consolidated description of the open roles, the hands-on expectations and the impact each role has on product quality and supply.
Quality Control — Officer / Sr. Officer (Ointment / RM / Intermediate / FP / FG — OSD & External Preparations)
Qualification: M.Sc (Chemistry) / B.Pharm
Experience: 2–5 years
Core responsibilities:
- Perform analytical testing of Finished Goods, Raw Materials and Intermediates across dosage forms (tablets, capsules, oral liquids, injectables and ointments).
- Operate and maintain instruments: HPLC, GC, Dissolution, Disintegration, Friability, UV spectrophotometer and related analytical equipment.
- Execute validated analytical methods, maintain calibration status and record instrument logs.
- Prepare and review test results, maintain GLP-compliant documentation and participate in OOS investigations.
Why this role matters: Accurate QC release testing prevents substandard batches from reaching the market and supports regulatory submissions and stability programs.
Quality Assurance — Assistant Manager (Lab QA)
Qualification: B.Pharm / M.Pharm
Experience: 8–10 years
Core responsibilities:
- Oversee the QC laboratory QMS, ensuring GLP and data integrity across all lab operations.
- Develop and implement QA controls focused on laboratory processes, calibration, method verification and staff training.
- Coordinate internal audits, support external inspections and ensure closure of CAPAs related to lab activities.
- Mentor laboratory staff on critical quality attributes, documentation practices and compliance expectations.
Why this role matters: Lab QA guarantees that QC outputs are reliable, auditable and defensible during regulatory reviews.
Manufacturing — Assistant Manager (Injectable & OSD Operations)
Qualification: M.Sc (Chemistry) / B.Pharm
Experience: 5–10 years depending on sub-function
Core responsibilities for Injectable Manufacturing:
- Supervise injectable production activities — charging, filtration, sterilization steps, aseptic handling and filling operations.
- Manage manpower allocation, reagent availability, and ensure batch documentation (BMR/BPR) accuracy.
- Support equipment troubleshooting, oversee plant utilities and handle in-process checks.
- Ensure adherence to aseptic practices and validation requirements.
Core responsibilities for OSD (Syrup/Suspension/FG & RM management):
- Lead daily production planning and batch execution for liquid and solid oral dosage forms.
- Monitor critical process parameters like mixing speed, filtration efficiency and suspension stability.
- Ensure availability of dispensed raw materials, purified water and utilities for uninterrupted production.
Why this role matters: Manufacturing leads translate protocols into compliant batches — they are the linchpin between process design and product availability.
Injectable — Dy. Manager / Asst. Manager (Liquid Injectables)
Qualification: B.Pharm / M.Pharm / B.Sc / M.Sc
Experience: 8–10 years
Core responsibilities:
- Manage sterile production lines, enforce environmental monitoring, and lead OOS investigations for injectables.
- Drive troubleshooting for equipment breakdown, ensure Elogbook entries are maintained and support validation activities.
- Lead cross-functional interactions with QA and QC for batch release and regulatory readiness.
Why this role matters: Injectable manufacturing demands elevated compliance — errors have higher patient-safety implications and stricter regulatory scrutiny.
FG/RM Warehouse — Executive
Qualification: B.Pharm / M.Pharm / B.Sc / M.Sc
Experience: 5–9 years
Core responsibilities:
- Manage daily warehouse operations: receiving, storage, picking, packing and dispatch of raw materials and finished goods.
- Maintain accurate inventory records through cycle counts and system updates; prepare reports on inventory KPIs.
- Coordinate with production and quality teams to ensure material availability and WMS compliance.
Why this role matters: Controlled warehouse operations preserve material integrity and ensure supply continuity.
Eligibility / Qualifications
Accepted degrees and relevant courses: M.Sc Chemistry, B.Pharm, M.Pharm Pharmaceutical Sciences, B.Sc Microbiology, Diploma in Industrial Pharmacy, B.Tech Chemical Engineering, PG Diploma in QA/QC, Certificate in HPLC Operations, Certification in GMP Practices
Experience range: 2–10 years depending on the role
Core competencies across roles:
- Strong analytical skills and hands-on instrument experience (HPLC/GC/Dissolution/Friability)
- Deep working knowledge of cGMP, QMS, documentation and OOS handling
- Ability to lead teams (for managerial roles) and support audits/inspections
- Clear written communication and accurate record-keeping
Application Process
Interested candidates should share their resume to recruitment@eristherapeutics.com or contact via WhatsApp at 9558001555 / 9414100208.
Application checklist:
- Updated CV with clear role-wise experience
- Educational certificates and training proof (if available)
- Short cover note (2–3 lines) summarising the most relevant experience for the applied role
FAQs
Q: Are freshers eligible?
No. These roles require prior industry experience as indicated per position.
Q: Which instruments should I list on my CV?
HPLC, GC, Dissolution, Disintegration, Friability, UV, pH meters, sterilization equipment and LIMS/WMS experience.
Q: Will I be required to support audits?
Yes. Several roles expect audit/inspection support and CAPA closure involvement.
Q: Is relocation required?
Location specifics will be shared during the interview; candidates should confirm willingness to relocate if needed.
Q: How do I improve my chances?
Provide concise, documented examples: one-page summary of a validation you led, an OOS investigation you helped close, or inventory accuracy improvements you implemented.
+----------------------+---------------------------------------------------------------+
| Company | Eris Therapeutics Limited (Subsidiary of Erislifesciences Ltd) |
+----------------------+---------------------------------------------------------------+
| Vacancies | QC Officer/Sr Officer, QA Asst Manager, Manufacturing AM, |
| | Dy.Manager (Injectables), FG/RM Executive |
+----------------------+---------------------------------------------------------------+
| Required Education | M.Sc Chemistry, B.Pharm, M.Pharm, B.Sc, B.Tech Chemical |
+----------------------+---------------------------------------------------------------+
| Experience | 2–10 years depending on role |
+----------------------+---------------------------------------------------------------+
To apply for this job email your details to recruitment@eristherapeutics.com


