Lifecare Neuro Hiring QA, QC, Production & Artwork

- B.Pharm/B.Sc OSD QA QC Openings – Baddi Plant
- Company Overview
- Job Role & Responsibilities
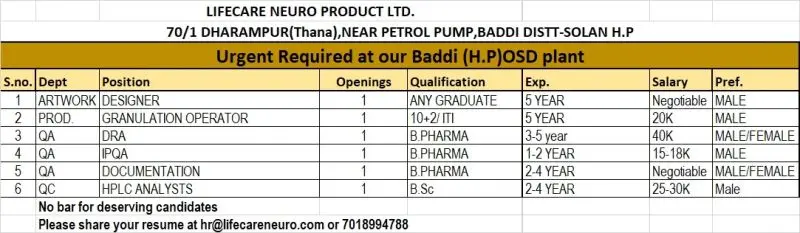
- 1. Artwork Designer
- 2. Production – Granulation Operator
- 3. Quality Assurance – DRA (Regulatory Documentation)
- 4. Quality Assurance – IPQA
- 5. Quality Assurance – Documentation
- 6. Quality Control – HPLC Analyst
- Eligibility / Qualifications
- Location & Salary
- Application Process
- FAQs
B.Pharm/B.Sc OSD QA QC Openings – Baddi Plant
Lifecare Neuro hiring for QA, QC, Production & Artwork roles at Baddi OSD plant. Multiple openings. Apply now.
Lifecare Neuro Product Ltd. is hiring skilled professionals for its OSD manufacturing plant in Baddi, Himachal Pradesh. The company is expanding its core Quality, Production and Artwork teams and looking for candidates with strong technical foundations, regulated manufacturing exposure and disciplined documentation habits. This hiring drive is ideal for those seeking stable growth in a GMP-focused environment.
Company Overview
Lifecare Neuro Product Ltd. is a well-established pharmaceutical manufacturer with a strong footprint in oral solid dosage (OSD) formulations. The organisation follows strict GMP systems, structured batch processes and documentation-led quality controls. Its Baddi facility supports large-scale production, quality testing and regulatory documentation for domestic and semi-regulated markets.
The company values consistency, process adherence and hands‑on skills critical for sustainable compliance.
Job Role & Responsibilities
1. Artwork Designer
Openings: 1
Qualification: Any Graduate
Experience: 5 years
Preferred: Male
Responsibilities:
- Create, update and review artwork for labels, cartons, foils and packaging materials.
- Ensure alignment with regulatory requirements and product specifications.
- Coordinate with QA, Production and Procurement for artwork approvals.
- Maintain organised version control and documentation.
2. Production – Granulation Operator
Openings: 1
Qualification: 10+2 / ITI
Experience: 5 years
Preferred: Male
Responsibilities:
- Operate granulation equipment (RMG, FBD, blender) as per SOP.
- Manage sieving, blending and related pre-compression activities.
- Follow batch records and maintain GMP discipline.
- Support housekeeping, changeovers and material handling.
3. Quality Assurance – DRA (Regulatory Documentation)
Openings: 1
Qualification: B.Pharm
Experience: 3–5 years
Preferred: Male/Female
Responsibilities:
- Prepare and manage regulatory documentation and dossiers.
- Support product permissions, renewals and regulatory queries.
- Ensure documentation accuracy and compliance with statutory requirements.
- Work with QA, QC and Production to compile technical files.
4. Quality Assurance – IPQA
Openings: 1
Qualification: B.Pharm
Experience: 1–2 years
Preferred: Male
Responsibilities:
- Conduct in-process checks during granulation, compression and packing.
- Review BMR/BPR entries and ensure GDP compliance.
- Perform line clearance, sampling and real-time documentation.
- Support deviation, change control and CAPA activities.
5. Quality Assurance – Documentation
Openings: 1
Qualification: B.Pharm
Experience: 2–4 years
Preferred: Male/Female
Responsibilities:
- Manage controlled documents including SOPs, BMR, BPR and specifications.
- Maintain document control systems and archival.
- Support audits and provide required documentation on request.
- Ensure compliance with GDP and version control.
6. Quality Control – HPLC Analyst
Openings: 1
Qualification: B.Sc
Experience: 2–4 years
Preferred: Male
Responsibilities:
- Perform HPLC analyses for RM, FP and stability samples.
- Manage instrument calibration, system suitability and logbooks.
- Follow GLP and ensure data integrity.
- Support OOS/OOT investigations.
Eligibility / Qualifications
Accepted education: Any Graduate, 10+2, ITI, B.Pharm, B.Sc
Relevant courses (comma-separated): B.Pharm, HPLC Certification, QC Analyst Training, GMP/GDP Training, ITI Mechanical, ITI Electrical, Packaging Design Course
Experience: 1–5 years depending on role.
Location & Salary
Location: Lifecare Neuro Product Ltd., 70/1 Dharampur (Thana), Near Petrol Pump, Baddi, Dist. Solan, Himachal Pradesh
Salary:
- Granulation Operator: ~20,000/month
- IPQA: 15,000–18,000/month
- HPLC Analyst: 25,000–30,000/month
- Other positions: Negotiable based on experience
No bar for deserving candidates.
Application Process
Interested candidates may submit resumes to:
- Email: hr@lifecareneuro.com
- WhatsApp: 7018994788
Required details:
- Total experience
- Current CTC
- Expected CTC
- Notice period
- Preferred interview date
FAQs
Q: Are freshers eligible?
No. Minimum 1–2 years depending on the position.
Q: Is there flexibility in salary?
Yes. Salary is negotiable for strong performers.
Q: What shifts are available?
Standard shifts; details shared during interview.
Q: Is OSD experience mandatory?
Preferred for QA/QC and production roles.
+----------------------+-------------------------------------------------------------+
| Company | Lifecare Neuro Product Ltd. |
+----------------------+-------------------------------------------------------------+
| Vacancies | Artwork, Granulation, QA DRA, QA IPQA, QA Documentation, |
| | QC HPLC Analyst |
+----------------------+-------------------------------------------------------------+
| Required Education | Any Graduate, 10+2, ITI, B.Pharm, B.Sc |
+----------------------+-------------------------------------------------------------+
| Experience | 1–5 years (depending on role) |
+----------------------+-------------------------------------------------------------+
To apply for this job email your details to hr@lifecareneuro.com


