Covalent Walk-in Production, Documentation and Solvent Recovery

- B.Sc/B.Pharm Production & Solvent Recovery Openings – Covalent
- Company Overview
- Job Role & Responsibilities
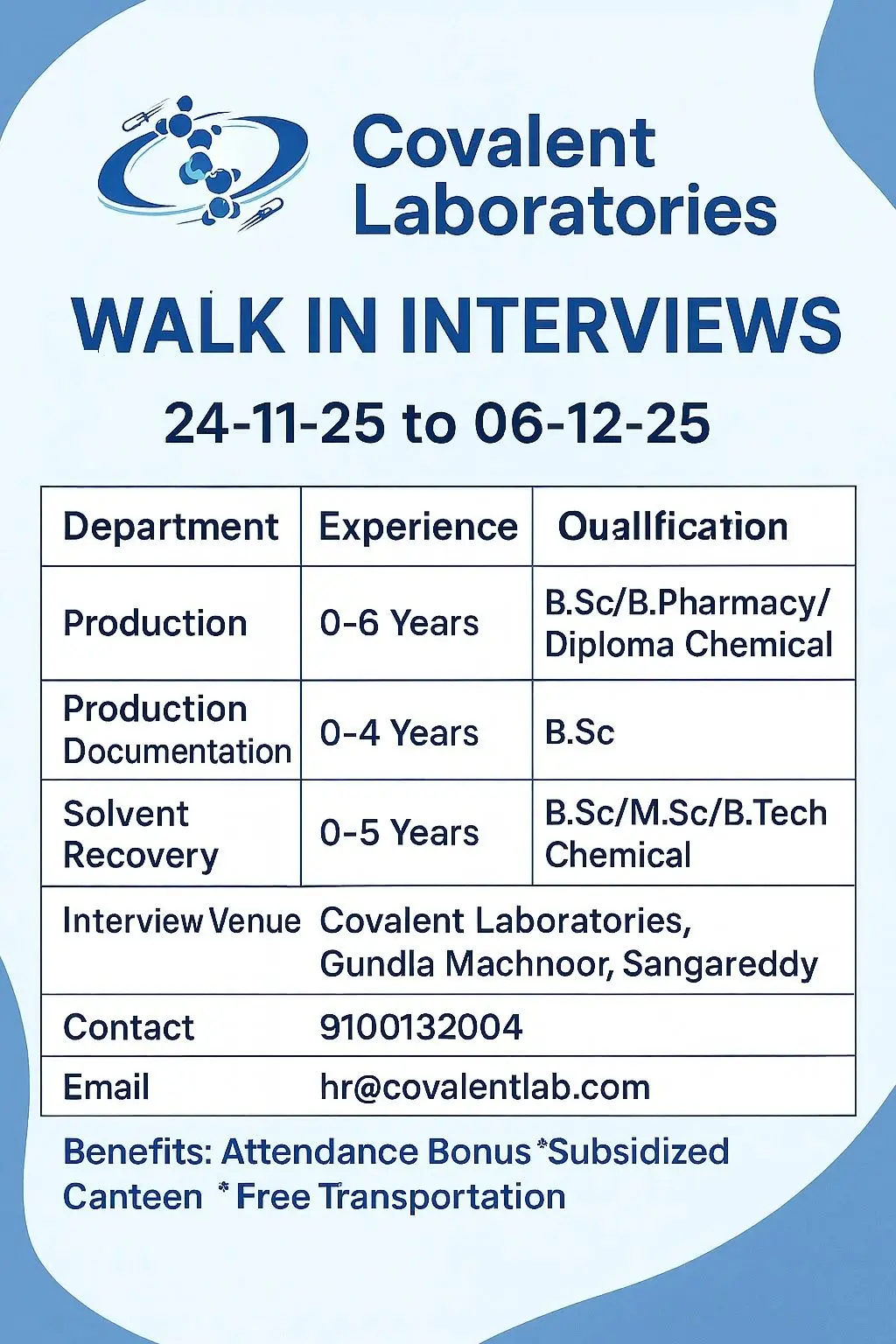
- Production – 0 to 6 Years
- Production Documentation – 0 to 4 Years
- Solvent Recovery – 0 to 5 Years
- Eligibility / Qualifications
- Required Education
- Experience
- Location & Salary
- Application Process
- Walk-In Interview Details
- FAQs
- Summary Table
B.Sc/B.Pharm Production & Solvent Recovery Openings – Covalent
Covalent Laboratories hiring B.Sc/B.Pharm/M.Sc/Diploma candidates for Production, Documentation and Solvent Recovery roles. Walk-in 24 Nov–6 Dec.
Covalent Laboratories is conducting walk-in interviews for multiple openings across Production, Production Documentation and Solvent Recovery at its Gundla Machnoor facility in Sangareddy. These roles suit freshers and experienced candidates with backgrounds in B.Sc, B.Pharm, M.Sc and Diploma in Chemical Engineering. The positions offer hands-on exposure to API manufacturing, solvent recovery operations, documentation control and GMP-driven production environments.
Company Overview
Covalent Laboratories is a growing API manufacturer known for its process reliability, cost-efficient operations and adherence to regulatory and quality standards. The company focuses on scalable manufacturing, strong documentation systems and safe chemical handling practices. With a steady footprint in the pharma sector, Covalent provides opportunities for technical growth and multi-discipline exposure.
Job Role & Responsibilities
Production – 0 to 6 Years
- Execute batch manufacturing operations as per SOPs.
- Handle reactors, centrifuges, dryers and filtration equipment.
- Monitor in-process parameters and maintain batch records.
- Follow GMP, safety procedures and equipment cleaning guidelines.
Production Documentation – 0 to 4 Years
- Prepare, review and update production-related documents.
- Maintain BMR/BPR accuracy and support audits.
- Track documentation discrepancies and coordinate with QA.
- Ensure controlled documentation practices and compliance.
Solvent Recovery – 0 to 5 Years
- Operate solvent recovery units and distillation systems.
- Maintain solvent purity checks and yield tracking.
- Monitor equipment condition and ensure safe chemical handling.
- Support environmental and waste-management compliance.
Eligibility / Qualifications
Required Education
- B.Sc (Chemistry, Biotechnology, Microbiology, Industrial Chemistry)
- B.Pharm (Pharmaceutics, Pharmaceutical Chemistry)
- M.Sc (Organic Chemistry, Analytical Chemistry, General Chemistry)
- Diploma Chemical Engineering
Experience
- Production: 0–6 years
- Production Documentation: 0–4 years
- Solvent Recovery: 0–5 years
These qualifications align with key API operations requiring GMP compliance, chemical handling, batch documentation and distillation process skills.
Location & Salary
Location: Covalent Laboratories, Gundla Machnoor, Sangareddy.
Salary will be based on experience, technical capability and departmental role. Benefits include:
- Attendance bonus
- Subsidized canteen
- Free transportation
Application Process
Walk-In Interview Details
Dates: 24 November 2025 to 6 December 2025
Venue: Covalent Laboratories, Gundla Machnoor, Sangareddy
Contact: 9100132004
Email: hr@covalentlab.com
Bring the following:
- Updated CV
- Educational certificates
- Government ID proof
- Experience letters (if applicable)
FAQs
Q1. Are freshers eligible?
Yes. Freshers can apply for Production, Documentation and Solvent Recovery roles.
Q2. What skills help in selection?
Basic chemistry knowledge, understanding of production equipment, documentation accuracy and willingness to work in shifts.
Q3. What is the interview process?
Walk-in screening, technical discussion and HR evaluation.
Q4. Are benefits provided?
Yes. Transportation, canteen subsidy and attendance bonus.
Q5. What industries are preferred?
API, chemical manufacturing and related process plants.
Summary Table
| Category | Details |
|---|---|
| Company | Covalent Laboratories |
| Vacancies | Production, Production Documentation, Solvent Recovery |
| Required Education | B.Sc, B.Pharm, M.Sc, Diploma Chemical Engineering |
| Experience | 0–6 years depending on department |