Lupin Walk-in Production & QC

- Pharma Production & QC Openings – Lupin Dabhasa
- Company Overview
- Job Role & Responsibilities
- Production – Jr. Officer / Officer
- Production – GMP Coordinator (Officer / Executive)
- Quality Control – Officer / Executive
- Eligibility / Qualifications
- Required Education
- Experience Requirements
- Additional Requirements
- Location & Salary
- Walk-In Interview Details
- Application Process
- FAQs
- Are freshers eligible?
- What regulatory exposure is preferred?
- Does the role involve shift work?
- What skills are important for GMP Coordinator roles?
- Can candidates from API plants apply?
- Summary Table
Pharma Production & QC Openings – Lupin Dabhasa
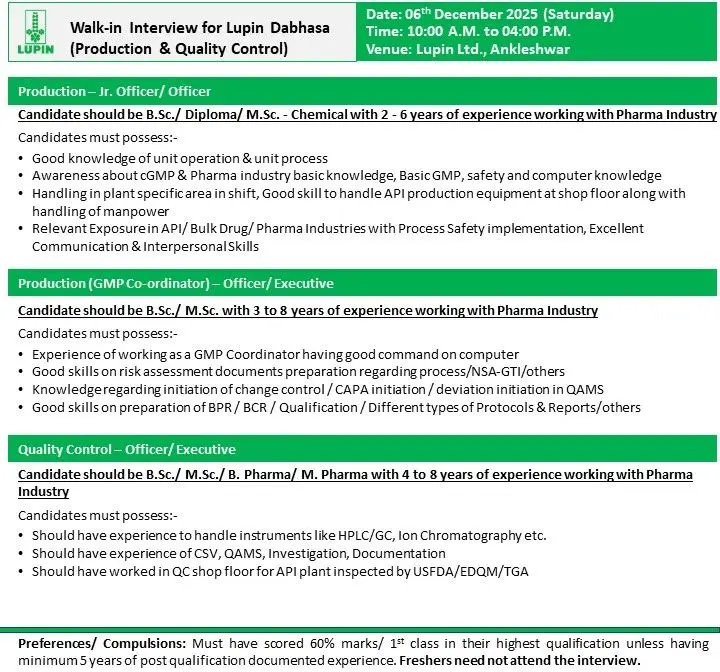
Lupin Dabhasa hiring BSc/MSc/BPharm candidates for Production & QC roles. Walk-in on 6 Dec 2025 at Ankleshwar.
Lupin Dabhasa is conducting a walk-in interview for multiple openings in Production and Quality Control. These roles are meant for skilled professionals with hands-on experience in API, bulk drugs, and regulated manufacturing environments. The openings cover junior to mid-level positions and require strong technical skills, sound GMP knowledge, and the ability to manage operations on the shop floor.
Company Overview
Lupin is one of India’s most respected pharmaceutical manufacturers, known for its FDA-approved facilities, strong compliance culture, and global presence in formulations and APIs. The Dabhasa site is a key manufacturing hub supporting large-scale API production, quality control operations, and technology-driven process improvements. The facility offers structured training, exposure to international regulatory standards, and a stable growth environment.
Job Role & Responsibilities
Openings are available across Production and Quality Control.
Production – Jr. Officer / Officer
Qualification: B.Sc / Diploma / M.Sc (Chemical)
Experience: 2–6 years
Key Responsibilities:
- Handle unit operations and unit processes at the shop floor.
- Operate API production equipment and support shift activities.
- Maintain GMP compliance, safety practices, and documentation accuracy.
- Perform in-plant operations including charging, filtration, drying, and related steps.
- Support manpower handling and coordination.
- Work with QA/QC for in-process checks and batch compliance.
- Follow process safety requirements and standard operating procedures.
Production – GMP Coordinator (Officer / Executive)
Qualification: B.Sc / M.Sc
Experience: 3–8 years
Key Responsibilities:
- Act as GMP coordinator to ensure site-wide compliance.
- Prepare risk assessment documents for processes, NSA-GTI, and related evaluations.
- Initiate and track change controls, deviations, and CAPA in QAMS.
- Prepare BPR/BCR, qualification protocols, reports, and technical documents.
- Support internal audits, data integrity checks, and regulatory readiness.
- Ensure continuous improvement of QMS processes.
Quality Control – Officer / Executive
Qualification: B.Sc / M.Sc / B.Pharm / M.Pharm
Experience: 4–8 years
Key Responsibilities:
- Operate and maintain QC instruments such as HPLC, GC, Ion Chromatography.
- Perform analysis for raw materials, intermediates, and APIs.
- Work with CSV systems, QAMS, documentation, and investigation processes.
- Support stability studies, impurity profiling, and analytical troubleshooting.
- Work in QC shop floor environments for API units inspected by USFDA/EDQM/TGA.
Eligibility / Qualifications
Required Education
- B.Sc (Chemistry/Science)
- M.Sc (Chemistry)
- Diploma (Chemical)
- B.Pharm / M.Pharm
Relevant courses: Industrial Chemistry, Organic Chemistry, Analytical Chemistry, Pharmaceutical Chemistry, Chemical Engineering.
Experience Requirements
- Production (Jr. Officer/Officer): 2–6 years
- Production (GMP Coordinator): 3–8 years
- Quality Control: 4–8 years
Additional Requirements
- Minimum 60% marks / First Class in highest qualification unless candidate has 5+ years relevant experience.
- Freshers should not attend.
- Strong communication and documentation skills.
Location & Salary
Walk-In Venue: Lupin Ltd., Ankleshwar
Job Location: Dabhasa Unit (Gujarat)
Salary will be based on experience, role seniority, and technical capability. QC and GMP roles supporting USFDA/EDQM/TGA compliance typically offer higher slabs due to regulatory importance.
Walk-In Interview Details
- Date: 06 December 2025 (Saturday)
- Time: 10:00 AM – 04:00 PM
- Venue: Lupin Ltd., Ankleshwar
Application Process
Walk-in directly with updated resume and supporting documents. Candidates must carry:
- Educational certificates
- Experience/relieving letters
- Pay slips
- Government ID
FAQs
Are freshers eligible?
No. Only experienced candidates should attend.
What regulatory exposure is preferred?
USFDA, EDQM, and TGA exposure is highly valued for QC roles.
Does the role involve shift work?
Yes. Production roles require shift handling.
What skills are important for GMP Coordinator roles?
Strong QMS understanding, documentation skills, QAMS familiarity, and risk assessment capability.
Can candidates from API plants apply?
Yes. Experience in API/Bulk Drug units is preferred.
Summary Table
| Category | Details |
|---|---|
| Company | Lupin Ltd. – Dabhasa |
| Vacancies | Production (Jr Officer/Officer, GMP Coordinator), QC Officer/Executive |
| Required Education | B.Sc, M.Sc, Diploma (Chemical), B.Pharm, M.Pharm |
| Experience | 2–8 years depending on role |