Macleods walk-in Quality Control , QA , Production , Technical Associate, Engineering

- Company Overview
- Job Role & Responsibilities
- Quality Control – Solid Oral Dosage (OSD)
- Engineering Services – Formulation Plants
- Production – Dry Powder Injection & Formulation
- Stores – API & Formulation Units
- Quality Assurance & API Quality Control
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Walk-In Details
- Why Consider This Opportunity at Macleods?
- Frequently Asked Questions (FAQs)
- Is this walk-in drive open for freshers?
- Is online registration mandatory?
- What departments are hiring?
- What documents should I carry?
- Is this role suitable for regulatory market exposure?
- SEO Title Suggestions
- Education Background – Eligible Courses
B.Pharm, M.Pharm, B.Sc Vacancies at Macleods – Ahmedabad Walk-In Drive
Macleods Pharmaceuticals walk-in drive at Ahmedabad for B.Pharm, M.Pharm, B.Sc, Diploma candidates. Multiple vacancies. Apply now.
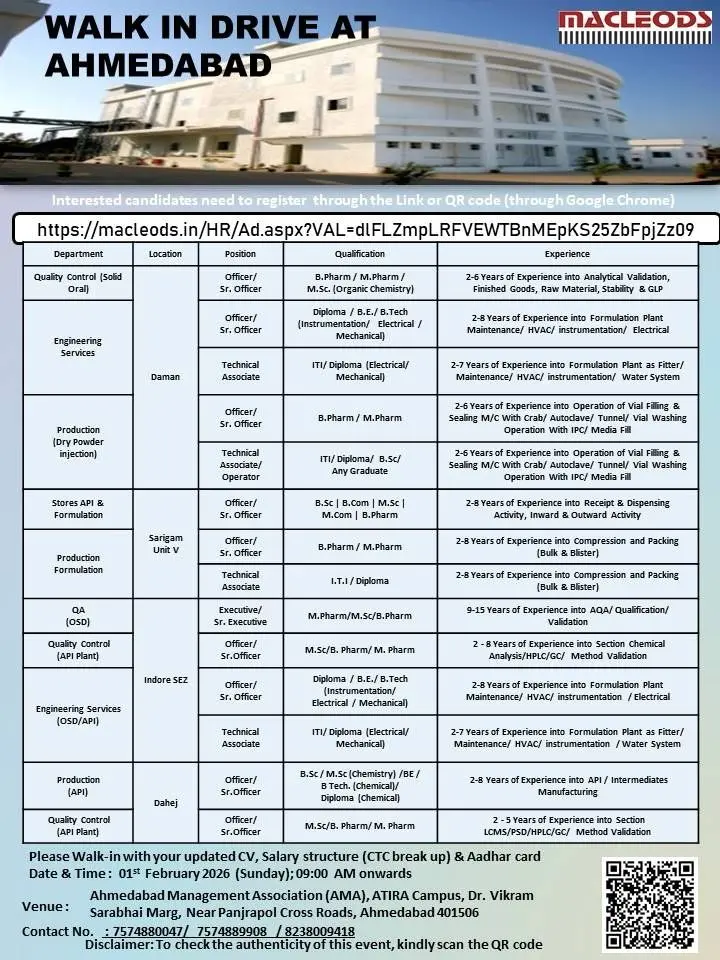
Macleods Pharmaceuticals is conducting a large-scale walk-in drive at Ahmedabad for experienced professionals across Quality Control, Quality Assurance, Production, Engineering Services, and Stores functions. This hiring initiative targets skilled candidates from pharma manufacturing, API plants, formulation units, and engineering services who are looking for stable, long-term careers with a reputed Indian pharmaceutical company. The drive offers multiple vacancies across locations such as Ahmedabad, Daman, Dahej, Sarigam, and Indore SEZ, making it a strong opportunity for candidates seeking pharma jobs with growth, compliance exposure, and technical learning.
Company Overview
Macleods Pharmaceuticals Ltd. is one of India’s fastest-growing pharmaceutical companies with a strong presence in regulated and semi-regulated markets. The company is known for its robust manufacturing infrastructure, compliance-driven quality systems, and focus on affordable healthcare solutions. Macleods operates multiple formulation and API manufacturing plants across India, adhering to global regulatory standards including WHO-GMP, USFDA, and EU-GMP.
With a diversified portfolio covering oral solids, injectables, APIs, and specialty products, Macleods provides strong career opportunities for professionals in pharmaceutical production, quality control, quality assurance, engineering, and supply chain operations. Working at Macleods allows professionals to contribute directly to safe, effective, and high-quality medicines that impact global healthcare.
Job Role & Responsibilities
Quality Control – Solid Oral Dosage (OSD)
Candidates selected for Quality Control roles will be responsible for analytical testing and quality evaluation of raw materials, finished products, and stability samples. Key responsibilities include:
• Analytical method validation and verification
• Finished goods and raw material analysis
• Stability studies and documentation
• GLP compliance and data integrity adherence
• Handling instruments such as HPLC, GC, and related analytical equipment
Engineering Services – Formulation Plants
Engineering professionals will support plant maintenance and utility operations to ensure uninterrupted manufacturing. Responsibilities include:
• Preventive and breakdown maintenance of formulation equipment
• HVAC system maintenance and monitoring
• Electrical, mechanical, and instrumentation maintenance
• Water system operation and upkeep
• Ensuring compliance with safety and GMP standards
Production – Dry Powder Injection & Formulation
Production professionals will handle day-to-day manufacturing activities in sterile and non-sterile areas. Responsibilities include:
• Operation of vial filling and sealing machines
• Autoclave, tunnel, and vial washing operations
• In-process checks and media fill activities
• Compression and packing operations (bulk and blister)
• Adherence to SOPs, GMP, and productivity targets
Stores – API & Formulation Units
Stores professionals will manage material movement and inventory control. Responsibilities include:
• Receipt and dispensing of raw materials and packing materials
• Inward and outward logistics activities
• Documentation and ERP-based inventory management
• Coordination with production and quality departments
• Ensuring compliance with GMP and traceability requirements
Quality Assurance & API Quality Control
Senior QA and QC professionals will handle advanced compliance and validation activities. Responsibilities include:
• AQA activities, qualification, and validation
• Method validation and technology transfer
• Chemical analysis using HPLC, GC, LCMS, PSD
• API and intermediates manufacturing support
• Regulatory compliance and audit readiness
Eligibility / Qualifications
The walk-in drive is open to candidates with the following educational backgrounds:
B.Pharm, M.Pharm, Pharm.D, B.Sc (Chemistry, Organic Chemistry), M.Sc (Chemistry), Diploma (Chemical, Electrical, Mechanical, Instrumentation), ITI (Fitter, Electrical, Mechanical), B.E, B.Tech, Any Graduate, B.Com, M.Com
Relevant experience requirements range from 2 to 15 years depending on the role and department. Candidates with prior experience in regulated pharma manufacturing environments will be preferred.
Location & Salary
Job locations include:
• Ahmedabad
• Daman
• Dahej
• Sarigam Unit V
• Indore SEZ
Salary will be offered as per industry standards and will be commensurate with qualifications, experience, and current CTC. Candidates are required to carry their CTC breakup during the walk-in process.
Application Process
Interested candidates must register online before attending the walk-in interview. Registration is mandatory and should be completed using Google Chrome for smooth access.
Apply Online (Mandatory Registration):
https://macleods.in/HR/Ad.aspx?VAL=dlFLZmpLRFVEWTBnMEpKS25ZbFpjZz09
After successful registration, candidates should walk in with the following documents:
• Updated resume
• Salary structure / CTC breakup
• Aadhaar card
Walk-In Details
Date: 01 February 2026 (Sunday)
Time: 09:00 AM onwards
Venue: Ahmedabad Management Association (AMA), ATIRA Campus, Dr. Vikram Sarabhai Marg, Near Panjrapol Cross Roads, Ahmedabad – 401506
Contact Numbers: 7574880047 / 7574889908 / 8238009418
Why Consider This Opportunity at Macleods?
• Employment with a trusted pharmaceutical brand
• Exposure to regulated manufacturing environments
• Strong learning and career growth opportunities
• Competitive salary packages
• Stable long-term employment
Frequently Asked Questions (FAQs)
Is this walk-in drive open for freshers?
No. This hiring drive is primarily for experienced professionals with a minimum of 2 years of relevant pharma industry experience.
Is online registration mandatory?
Yes. Candidates must complete online registration before attending the walk-in interview.
What departments are hiring?
Quality Control, Quality Assurance, Production, Engineering Services, and Stores across OSD, API, and Injectables.
What documents should I carry?
Updated CV, CTC breakup, and Aadhaar card are mandatory.
Is this role suitable for regulatory market exposure?
Yes. Macleods operates regulated facilities, offering strong exposure to compliance and audit standards.
SEO Title Suggestions
Macleods Pharmaceuticals Walk-In Drive 2026 – QC, QA, Production & Engineering Vacancies
Education Background – Eligible Courses
B.Pharm, M.Pharm, Pharm.D, B.Sc Chemistry, B.Sc Organic Chemistry, M.Sc Chemistry, Diploma Chemical Engineering, Diploma Mechanical Engineering, Diploma Electrical Engineering, Diploma Instrumentation, ITI Fitter, ITI Electrical, B.E Mechanical, B.Tech Chemical, Any Graduate
| Company | Macleods Pharmaceuticals Ltd. |
|---|---|
| Vacancies | Quality Control Officer, Senior Officer, QA Executive, Production Officer, Technical Associate, Engineering Officer |
| Required Education | B.Pharm, M.Pharm, B.Sc, M.Sc, Diploma, ITI, B.E, B.Tech |
| Experience | 2 to 15 Years |
To apply for this job please visit macleods.in.


