Anthea Pharma Hiring Quality Assurance Validation Executive/Sr. Executive
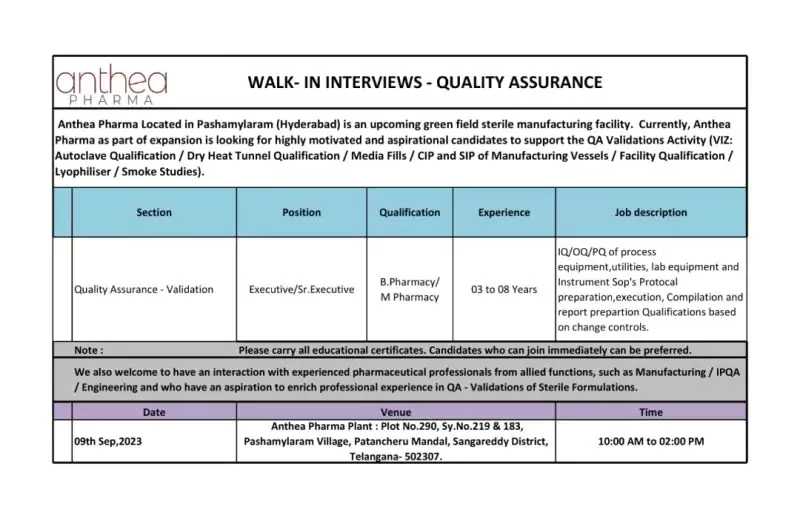
Anthea Pharma, located in Pashamylaram (Hyderabad), is a rapidly growing greenfield sterile manufacturing facility. We are committed to ensuring the highest standards of quality in pharmaceutical manufacturing. As part of our expansion plans, we are seeking dedicated and driven candidates to join our Quality Assurance Validation team. If you are passionate about pharmaceutical quality and have the skills and experience in validation activities, we invite you to explore this exciting opportunity.
Position Title: Quality Assurance Validation Executive/Sr. Executive
Company Name: Anthea Pharma
Salary: Competitive salary based on experience
Company Address: Anthea Pharma Plant, Plot No.290, Sy.No.219 & 183, Pashamylaram Village, Patancheru Mandal, Sangareddy District, Telangana-502307.
Detailed Job Description
Role: Quality Assurance – Validation
Industry Type: Pharmaceutical
Department: Quality Assurance
Employment Type: Full-Time
Role Category: Pharmaceutical Validation
Education
UG: B Pharmacy/M.Pharmacy
PG: [Optional]
Key Skills: IQ/OQ/PQ, Autoclave Qualification, Dry Heat Tunnel Qualification, Media Fills, CIP and SIP of Manufacturing Vessels, Facility Qualification, Lyophilizer, Smoke Studies, Equipment Validation, Utilities Validation, Lab Equipment Validation, SOPs, Change Control Qualifications
Job Description:
As an Executive/Sr. Executive in Quality Assurance Validation at Anthea Pharma, you will be responsible for a range of critical validation activities, including but not limited to:
- IQ/OQ/PQ of Process: Execute Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) of various processes.
- Equipment and Utilities Validation: Ensure validation of essential equipment and utilities required for pharmaceutical manufacturing.
- Lab Equipment Validation: Validate laboratory equipment to maintain the highest quality control standards.
- Instrument SOPs: Develop and manage Standard Operating Procedures (SOPs) for instruments used in quality assurance.
- Protocol Preparation and Execution: Create comprehensive validation protocols, execute them, and compile data for thorough validation reports.
- Change Control Qualifications: Implement validation activities based on change controls to ensure ongoing compliance.
Note:
- Candidates are requested to bring all educational certificates for the interview.
- Candidates with the ability to join immediately will be given preference.
- We also encourage experienced pharmaceutical professionals from related functions, including Manufacturing, In-Process Quality Assurance (IPQA), and Engineering, who aspire to enhance their expertise in QA – Validations of Sterile Formulations, to connect with us.
Date: 9th September 2023
Time: 10:00 AM to 02:00 PM
Walk-in-interview Address: Anthea Pharma Plant, Plot No.290, Sy.No.219 & 183, Pashamylaram Village, Patancheru Mandal, Sangareddy District, Telangana-502307.