BDR hiring Regulatory Affairs Executives & Managers for API DMF

- Company Overview
- Job Role & Responsibilities
- Regulatory Affairs – API (Global Markets)
- Eligibility / Qualifications
- Required Education
- Experience Requirements
- Location & Salary
- Application Process
- Why Join BDR Pharmaceuticals
- SEO-Optimized Job Titles
- Frequently Asked Questions (FAQs)
- Who can apply for BDR Regulatory Affairs API roles?
- Which markets does this role support?
- Is API regulatory experience mandatory?
- Where is the job location?
- How can I apply?
- Summary Table
MSc Regulatory Affairs API Jobs at BDR Pharma Vadodara
BDR Pharmaceuticals hiring Regulatory Affairs Executives & Managers for API DMF roles at Luna Plant, Vadodara.
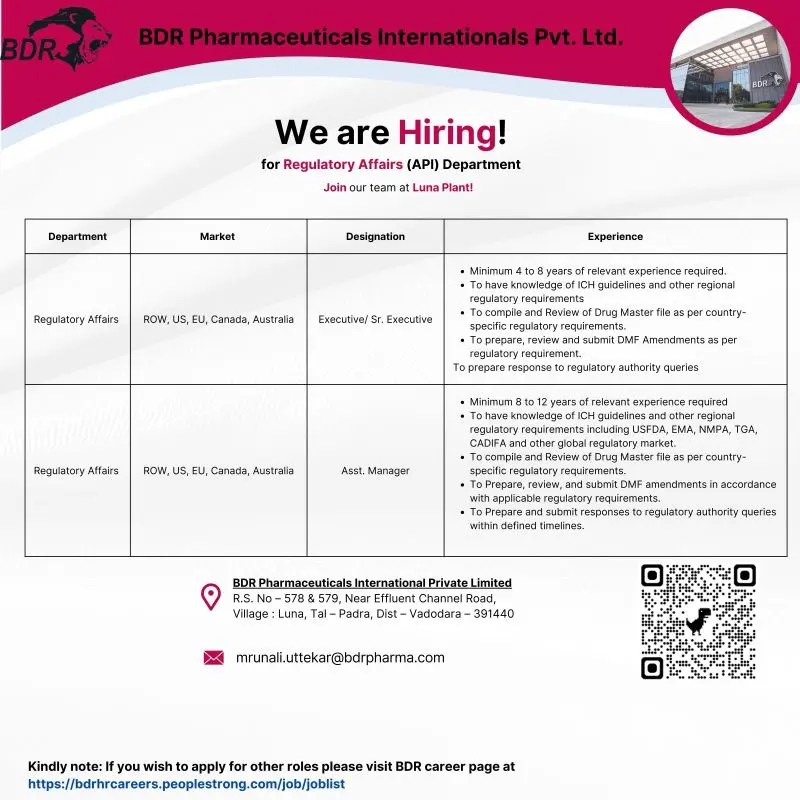
BDR Pharmaceuticals International Private Limited has announced multiple openings in its Regulatory Affairs (API) department at the Luna manufacturing plant near Vadodara. This hiring drive is aimed at experienced regulatory professionals with strong exposure to global markets including ROW, US, EU, Canada, and Australia. The roles focus on Drug Master File (DMF) preparation, lifecycle management, and regulatory correspondence for API dossiers, offering an opportunity to work in a globally regulated pharmaceutical environment.
Company Overview
BDR Pharmaceuticals International Private Limited is a globally recognized pharmaceutical company specializing in Active Pharmaceutical Ingredients (APIs), oncology products, and complex generics. With a strong presence across regulated markets, BDR Pharma is known for its compliance-driven culture, scientific rigor, and robust regulatory strategies aligned with international standards.
The company’s Luna Plant in Vadodara supports API manufacturing and regulatory submissions for multiple global health authorities such as USFDA, EMA, TGA, Health Canada, and other international agencies. BDR Pharmaceuticals continues to expand its regulatory footprint through strong DMF portfolios and lifecycle management, making it a preferred employer for regulatory affairs professionals.
Job Role & Responsibilities
Regulatory Affairs – API (Global Markets)
Executive / Senior Executive – Regulatory Affairs
Experience: 4 to 8 Years
Key Responsibilities:
- Compiling and reviewing Drug Master Files (DMFs) as per country-specific regulatory requirements
- Preparing and submitting DMF amendments in accordance with global regulatory guidelines
- Handling regulatory submissions for ROW, US, EU, Canada, and Australia markets
- Preparing responses to regulatory authority queries within defined timelines
- Ensuring compliance with ICH guidelines and applicable regional regulations
- Coordinating with internal stakeholders for regulatory data compilation and review
Assistant Manager – Regulatory Affairs
Experience: 8 to 12 Years
Key Responsibilities:
- Managing end-to-end regulatory strategy for API DMFs across global markets
- Reviewing and approving DMF submissions and lifecycle amendments
- Handling regulatory communications with authorities such as USFDA, EMA, NMPA, TGA, Health Canada, and CADIFA
- Preparing, reviewing, and submitting responses to regulatory queries
- Providing regulatory guidance to cross-functional teams
- Ensuring adherence to global regulatory requirements and timelines
Eligibility / Qualifications
Required Education
- M.Sc (Chemistry)
- M.Pharm
Relevant Courses Include:
M.Sc Chemistry, M.Sc Pharmaceutical Chemistry, M.Pharm Pharmaceutics, M.Pharm Pharmaceutical Analysis, M.Pharm Regulatory Affairs
Experience Requirements
- Minimum 4–8 years for Executive / Senior Executive roles
- Minimum 8–12 years for Assistant Manager roles
- Hands-on experience in API regulatory affairs and DMF submissions
- Strong knowledge of ICH guidelines and global regulatory requirements
Location & Salary
Job Location:
BDR Pharmaceuticals International Private Limited
R.S. No. 578 & 579,
Near Effluent Channel Road,
Village: Luna, Taluka Padra,
District Vadodara – 391440
Salary Package:
Compensation will be competitive and aligned with industry benchmarks, based on experience and role level.
Application Process
Interested and eligible candidates can apply by sharing their updated resume via email or through the official career portal.
Email ID: mrunali.uttekar@bdrpharma.com
Career Page: https://bdrhrcareers.peoplestrong.com/job/joblist
Candidates are advised to mention the applied position in the email subject line.
Why Join BDR Pharmaceuticals
- Exposure to global regulatory submissions across multiple markets
- Strong DMF portfolio and API-focused regulatory work
- Opportunity to work with leading international regulatory authorities
- Structured growth path in regulatory affairs
- Compliance-driven and research-oriented work culture
SEO-Optimized Job Titles
- BDR Pharmaceuticals Regulatory Affairs Jobs
- API Regulatory Affairs DMF Vacancies
- Pharma Regulatory Jobs USFDA EMA Markets
- Regulatory Affairs Executive Jobs Vadodara
Frequently Asked Questions (FAQs)
Who can apply for BDR Regulatory Affairs API roles?
Candidates with relevant regulatory affairs experience in API DMF submissions can apply.
Which markets does this role support?
The roles support ROW, US, EU, Canada, Australia, and other global markets.
Is API regulatory experience mandatory?
Yes. Prior experience in API regulatory affairs and DMF handling is required.
Where is the job location?
The position is based at the Luna Plant near Vadodara, Gujarat.
How can I apply?
You can apply by emailing your resume or through the official BDR career portal.
Summary Table
| Company | BDR Pharmaceuticals International Private Limited |
|---|---|
| Vacancies | Executive / Sr. Executive – Regulatory Affairs, Assistant Manager – Regulatory Affairs |
| Required Education | M.Sc, M.Pharm |
| Experience | 4 to 12 Years |
To apply for this job please visit bdrhrcareers.peoplestrong.com.


