Concord Biotech Walk-in QC Micro, QA, Production and Purchase
- BSc/MSc Injectable QC & QA Openings – Concord Biotech Ahmedabad
- Company Overview
- Job Role & Responsibilities
- QC Microbiology – Trainee Officer to Asst. Manager
- Production – Aseptic Area / Dry Powder / Lyophilizer / Clean Area
- QA – Qualification (Executive/Sr. Executive)
- QA – IPQA (Officer)
- Purchase – Officer
- Eligibility / Qualifications
- Required Education
- Experience
- Location & Salary
- Application Process
- Walk-In Interview Details
- Required Documents
- FAQs
- Summary Table
BSc/MSc Injectable QC & QA Openings – Concord Biotech Ahmedabad
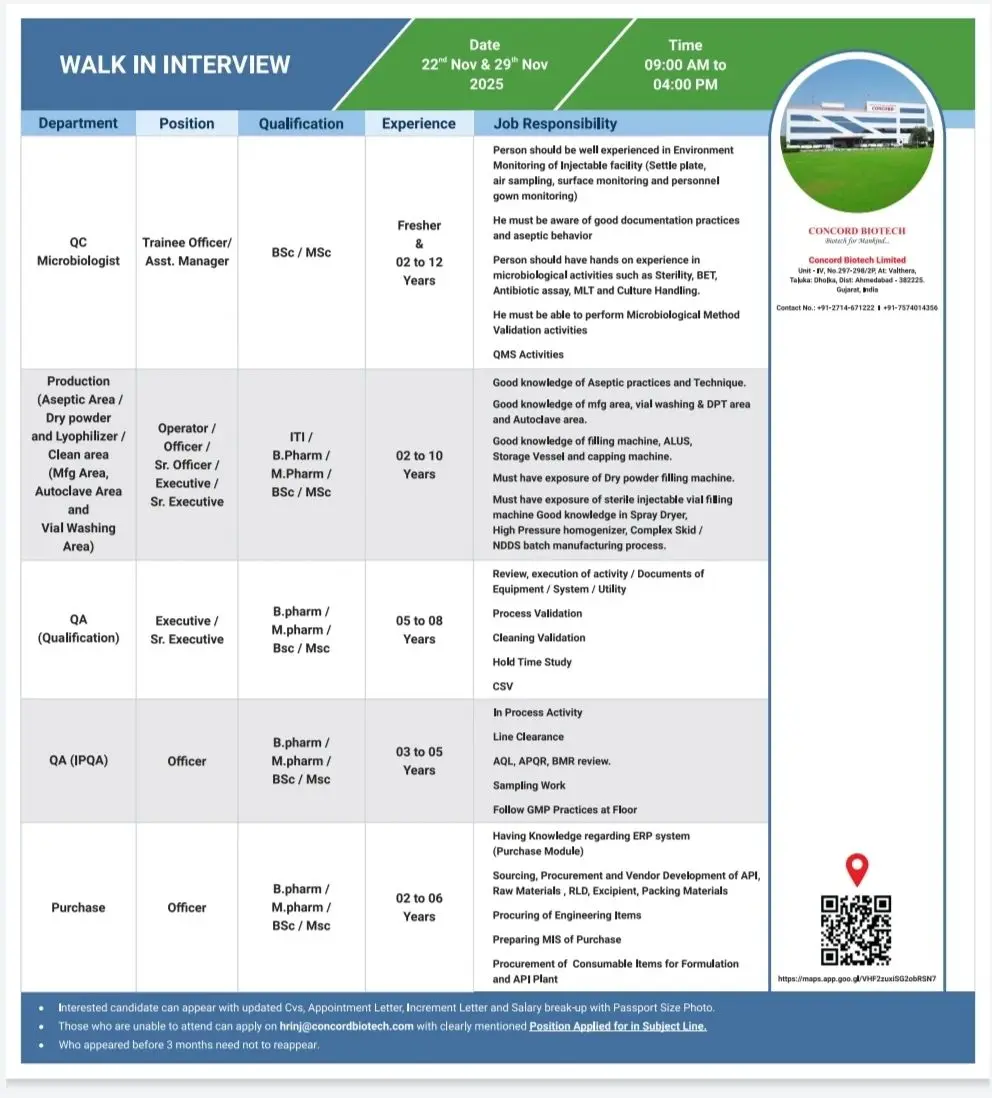
Concord Biotech hiring BSc/MSc/B.Pharm/M.Pharm candidates for QC Micro, QA, Production and Purchase roles. Walk-in on 22 & 29 Nov 2025.
Concord Biotech is conducting a walk-in interview for multiple roles across QC Microbiology, Production (Injectables, Dry Powder, Lyophilization), QA (Qualification & IPQA) and Purchase. These openings cover freshers and experienced candidates with backgrounds ranging from BSc and MSc to B.Pharm, M.Pharm and ITI. The positions suit applicants aiming to work in a regulated injectable facility with exposure to aseptic operations, microbiology testing, QMS, validation and procurement activities.
Company Overview
Concord Biotech Limited is a leading biopharmaceutical manufacturer with expertise in fermentation-based APIs and advanced sterile formulations. The company operates globally, supplying high-quality products to regulated markets. With strong R&D capabilities, advanced manufacturing units and a focus on quality systems, Concord Biotech offers a professional environment for candidates aiming to build long-term pharma careers.
Job Role & Responsibilities
Concord is hiring for QC Microbiology, Production, QA (Qualification & IPQA) and Purchase functions. Responsibilities vary by department.
QC Microbiology – Trainee Officer to Asst. Manager
- Perform sterility testing, BET, MLT, antibiotic assay and routine microbiology operations.
- Conduct microbiological method validation activities.
- Carry out environment monitoring: settle plates, active air sampling, surface monitoring and gowning checks.
- Follow aseptic technique, documentation standards and GLP practices.
Production – Aseptic Area / Dry Powder / Lyophilizer / Clean Area
Roles: Operator, Officer, Sr. Officer, Executive, Sr. Executive
- Operate and monitor aseptic filling, vial washing, DPT, autoclave and cleanroom equipment.
- Work with sterile vial filling machines, capping machines, ALUS, storage vessels and dry powder filling machines.
- Support activities involving spray dryers, high-pressure homogenizers, and complex skid/NDDS batch processes.
- Maintain GMP compliance and support routine manufacturing documentation.
QA – Qualification (Executive/Sr. Executive)
- Manage review and execution of qualification, validation and utility system activities.
- Support process validation, cleaning validation and hold-time studies.
- Execute CSV activities and maintain audit-ready documentation.
- Handle equipment/system protocols, reports and QMS activities.
QA – IPQA (Officer)
- Perform in-process checks, line clearance and sampling.
- Review BMRs, APQR and other GMP-controlled records.
- Ensure floor compliance and adherence to SOPs.
Purchase – Officer
- Manage procurement of APIs, raw materials, excipients, packaging items and consumables.
- Handle sourcing, vendor development, RLD procurement and engineering item purchasing.
- Maintain MIS and operate ERP (Purchase Module).
Eligibility / Qualifications
Required Education
- BSc, MSc (Microbiology, Biotechnology, Chemistry, Biochemistry)
- B.Pharm, M.Pharm (Pharmaceutics, Industrial Pharmacy, Pharmaceutical Technology)
- ITI (Multiple trades relevant to sterile production)
Experience
- Freshers and 2 to 12 years depending on role.
- Production: 2–10 years
- QA (Qualification): 5–8 years
- QA (IPQA): 3–5 years
- Purchase: 2–6 years
These profiles support high-demand pharma operations in sterile injectable manufacturing, microbiology testing and GMP-compliant quality systems.
Location & Salary
Location: Concord Biotech Limited, Unit-IV, Valthera, Dholka, Ahmedabad, Gujarat – 382225. Salary will be based on qualification, experience and department.
Application Process
Walk-In Interview Details
Dates: 22 November 2025 & 29 November 2025
Time: 9:00 AM – 4:00 PM
Venue: Concord Biotech Limited Unit-IV, No. 297-298/28, Valthera, Dholka, Ahmedabad – 382225
Required Documents
- Updated CV
- Appointment letter
- Increment letter
- Salary breakup
- Passport size photo
Candidates who attended interviews within the last 3 months need not reapply.
Email: hrinj@concordbiotech.com
Contact: +91-2714-671222, +91-7574014306
FAQs
1. Who can apply for QC Microbiology roles?
BSc/MSc candidates in Microbiology, Biotechnology or related fields.
2. Are freshers eligible?
Yes. Certain QC and Production roles accept freshers.
3. What experience is needed for QA Qualification?
5–8 years in qualification, validation and equipment/system documentation.
4. Is this a walk-in or online hiring?
Walk-in on 22 & 29 November 2025.
5. What skills are valued for Production roles?
Experience in aseptic operations, filling machines, autoclaves, dry powder filling and lyophilization.
Summary Table
| Category | Details |
|---|---|
| Company | Concord Biotech Limited |
| Vacancies | QC Micro, Production, QA (Qualification/IPQA), Purchase |
| Required Education | BSc, MSc, B.Pharm, M.Pharm, ITI |
| Experience | Fresher to 12 years depending on department |