Ravenbhel Biotech Walk-In QC, QA, Production Officers

- Ravenbhel Biotech Walk-In: QC, QA, Production Officers | Dehradun
- Company Overview
- Job Roles & Responsibilities
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join Ravenbhel Healthcare?
- Frequently Asked Questions (FAQs)
Ravenbhel Biotech Walk-In: QC, QA, Production Officers | Dehradun
Ravenbhel Healthcare hiring QC, QA, Production & Engineering Officers in Dehradun. Walk-in on 16 Nov 2025 with B.Pharm/M.Sc/B.Tech qualifications.
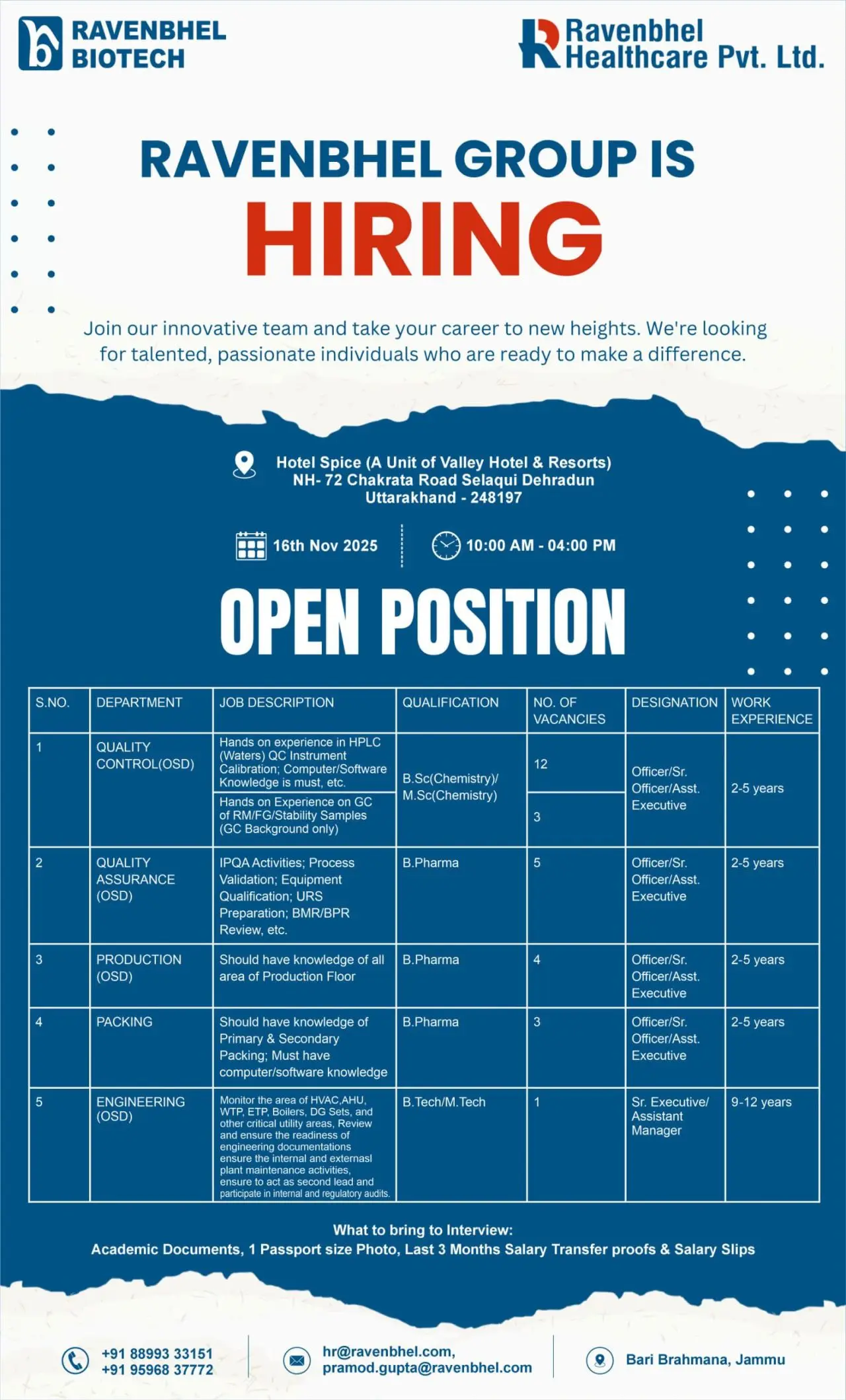
Ravenbhel Healthcare Pvt. Ltd., a flagship company under the Ravenbhel Group, is conducting a Walk-In Interview for multiple departments including Quality Control, Quality Assurance, Production, Packing, and Engineering. The drive is scheduled for 16 November 2025 in Dehradun (Uttarakhand). This is a remarkable opportunity for experienced pharma professionals to join a rapidly growing organization known for its commitment to innovation, compliance, and healthcare excellence.
Walk-In Details:
Date: 16th November 2025
Time: 10:00 AM to 04:00 PM
Venue: Hotel Spice (A Unit of Valley Hotel & Resorts), NH-72, Chakrata Road, Selaqui, Dehradun, Uttarakhand – 245157
Company Overview
Ravenbhel Healthcare Pvt. Ltd., part of the Ravenbhel Biotech Group, is a leading name in the Indian pharmaceutical industry. With over two decades of experience in manufacturing, formulation, and research, the company delivers high-quality and affordable medicines across multiple therapeutic areas. Ravenbhel has a strong presence in domestic and international markets and operates WHO-GMP certified facilities ensuring compliance with global quality and regulatory standards.
The company’s mission is to advance healthcare through innovation, continuous improvement, and sustainable growth. Employees at Ravenbhel are part of a forward-thinking culture that encourages scientific excellence, professional development, and global exposure.
Job Roles & Responsibilities
Quality Control (QC)
Designation: Officer / Sr. Officer / Assistant Executive
Experience: 2–5 years
Qualification: M.Sc (Chemistry) / B.Pharm
Key Responsibilities:
- Perform analysis of raw materials, intermediates, and finished products using HPLC and GC systems.
- Maintain laboratory documentation as per cGMP and GLP standards.
- Conduct stability testing, assay validation, and method transfers.
- Handle analytical data integrity, calibration, and troubleshooting.
Preferred Skills: Strong understanding of chromatographic techniques, analytical method validation, and regulatory audit handling.
Quality Assurance (QA)
Designation: Officer / Sr. Officer / Assistant Executive
Experience: 2–4 years
Qualification: B.Pharm / M.Sc
Key Responsibilities:
- Execute process validation, equipment qualification, URS preparation, and BMR/BPR review.
- Manage deviation, CAPA, and change control documentation.
- Perform internal audits and ensure compliance with cGMP and SOPs.
- Oversee QMS activities and support external inspections.
Preferred Skills: Knowledge of regulatory guidelines, validation protocols, and e-documentation systems.
Production (OSD)
Designation: Officer / Sr. Officer / Assistant Executive
Experience: 2–5 years
Qualification: B.Pharm / M.Pharm
Key Responsibilities:
- Supervise manufacturing operations across tablet, capsule, and oral dosage forms.
- Monitor process parameters and in-process quality checks.
- Ensure batch documentation accuracy and compliance with manufacturing SOPs.
- Coordinate with QA/IPQA for line clearance and validation.
Preferred Skills: In-depth understanding of OSD production equipment, GMP operations, and process optimization.
Packing Department
Designation: Officer / Sr. Officer / Assistant Executive / Manager
Experience: 2–12 years
Qualification: B.Pharm / M.Pharm / Graduate
Key Responsibilities:
- Oversee primary and secondary packing activities.
- Handle line clearance, batch reconciliation, and labeling verification.
- Maintain packing documentation and compliance with QA standards.
- Experience in Track & Trace, serialization, and packaging machinery is an advantage.
Preferred Skills: Practical knowledge of pharma packing lines, GMP, and data integrity principles.
Engineering Department
Designation: Officer / Sr. Officer / Assistant Executive
Experience: 2–5 years
Qualification: B.Tech / Diploma (Mechanical / Electrical / Industrial)
Key Responsibilities:
- Handle preventive and breakdown maintenance for production and utility equipment.
- Oversee operation and maintenance of HVAC, compressed air, water, and electrical systems.
- Support installation qualification (IQ), operation qualification (OQ), and equipment validation.
- Ensure safe working conditions and compliance with engineering standards.
Preferred Skills: Expertise in utilities management, documentation, and automation systems.
Eligibility / Qualifications
Educational Backgrounds Accepted: B.Pharm, M.Pharm, M.Sc (Chemistry), B.Tech, Diploma
Relevant Courses: Pharmaceutical Sciences, Industrial Pharmacy, Analytical Chemistry, Mechanical Engineering, Electrical Engineering
Experience Range: 2–12 years depending on role and designation
Core Competencies:
- Knowledge of GMP, GLP, QMS, and regulatory compliance.
- Hands-on experience in pharma formulation and analysis.
- Strong communication, problem-solving, and teamwork skills.
Location & Salary
Work Location: Selaqui, Dehradun, Uttarakhand
Salary: Competitive and commensurate with experience
Work Environment: Modern facilities with WHO-GMP certification and advanced production infrastructure.
Application Process
Interested candidates may attend the Walk-In Interview with the following documents:
- Updated Resume
- Academic Certificates
- One Passport-size Photograph
- Last 3 Months’ Salary Slips / Transfer Proofs
Email IDs: hr@ravenbhel.com | pramod.gupta@ravenbhel.com
Contact: +91 88993 33151
Corporate Office: Bari Brahmana, Jammu
Why Join Ravenbhel Healthcare?
- Opportunity to work with one of India’s leading pharmaceutical manufacturing organizations.
- Exposure to advanced technology, process automation, and regulatory systems.
- Great platform for career growth with supportive leadership and mentorship.
- Dynamic work culture encouraging innovation, collaboration, and learning.
- Contribute to global healthcare advancement through quality-driven pharmaceutical manufacturing.
Frequently Asked Questions (FAQs)
Q1. Who can apply for the Ravenbhel Healthcare Walk-In 2025?
Candidates with qualifications such as B.Pharm, M.Pharm, M.Sc (Chemistry), B.Tech, or Diploma and 2–12 years of relevant experience.
Q2. What is the date and venue of the walk-in interview?
The walk-in is on 16 November 2025 at Hotel Spice, NH-72, Chakrata Road, Selaqui, Dehradun.
Q3. Are freshers eligible for these vacancies?
No, all positions require a minimum of 2 years of experience in the pharmaceutical industry.
Q4. What should I bring to the interview?
Bring your resume, academic documents, photograph, and last three salary proofs.
Q5. Is HPLC experience mandatory for QC roles?
Yes, HPLC and GC operation knowledge are essential for QC positions.
| Category | Details |
|---|---|
| Company | Ravenbhel Healthcare Pvt. Ltd. (Ravenbhel Biotech Group) |
| Vacancies | QC, QA, Production, Packing, Engineering |
| Required Education | B.Pharm, M.Pharm, M.Sc (Chemistry), B.Tech, Diploma |
| Experience | 2–12 years |
| Location | Selaqui, Dehradun, Uttarakhand |