Pharmacovigilance Multiple Hiring at Vizen Life Sciences | Hyderabad

- Pharmacovigilance Careers – Vizen Life Sciences | Hyderabad
- Company Overview
- Job Role & Responsibilities
- Eligibility / Qualifications
- Location & Work Type
- Application Process
- Why Join Vizen Life Sciences?
- FAQs
Pharmacovigilance Careers – Vizen Life Sciences | Hyderabad
Apply for Pharmacovigilance roles at Vizen Life Sciences, Hyderabad. B.Pharm/M.Pharm/Pharm.D graduates with PV and ICSR experience welcome.
Advance your career in pharmacovigilance and drug safety operations with Vizen Life Sciences, a leading organization in clinical safety and regulatory compliance. Join our Hyderabad office and play a key role in ICSR processing, data entry, quality review, and medical case analysis to ensure patient safety and regulatory adherence.
Company Overview
Vizen Life Sciences Pvt Ltd is a recognized pharmacovigilance and drug safety service provider committed to maintaining high standards of regulatory compliance and clinical data quality. With a focus on integrity, innovation, and excellence, Vizen partners with pharmaceutical companies to manage adverse event reporting, safety data analysis, and regulatory submissions efficiently.
Our Hyderabad-based team brings together experienced professionals in ICSR management, medical review, and regulatory reporting, ensuring the highest level of pharmacovigilance and drug safety services.
Job Role & Responsibilities
Vizen Life Sciences offers multiple high CPC pharmacovigilance roles across different levels of experience:
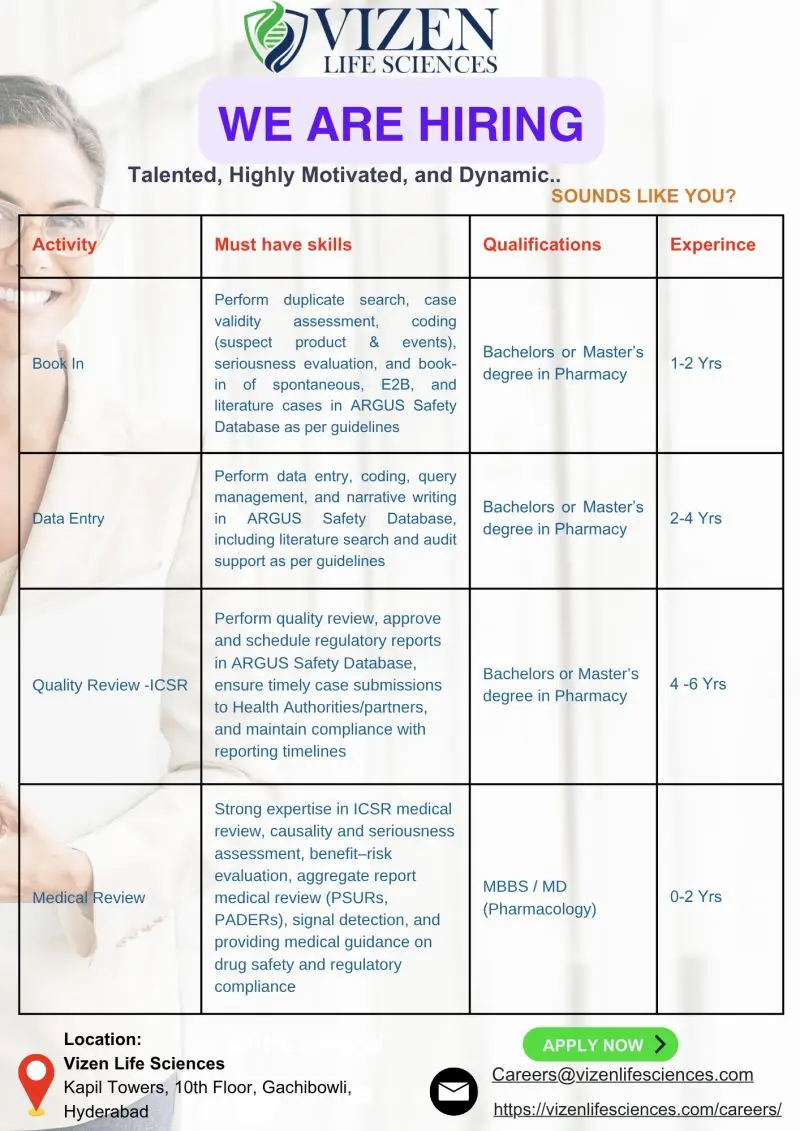
1. ICSR Book-In / Data Entry (1–3 Years)
- Conduct duplicate search and case validity assessment.
- Code suspect product events and MedDRA coding of adverse events.
- Perform book-in of spontaneous, E2B, and literature cases in ARGUS Safety Database.
- Manage query resolution and narrative writing.
- Conduct literature searches and audit support.
- Ensure compliance with PV regulations and reporting timelines.
2. Quality Review & Regulatory Report Submission (4–6 Years)
- Approve and schedule regulatory reports in ARGUS for submission to Health Authorities.
- Ensure adherence to internal and external timelines.
- Perform quality review of ICSRs including causality, seriousness, and completeness.
- Maintain GxP compliance and documentation standards.
3. Drug Safety Physician (MBBS / MD Pharmacology)
- Conduct ICSR medical review, causality and seriousness assessment.
- Write safety sections for Aggregate Reports (PSURs, CASRs, PADERs).
- Provide guidance to Drug Safety Associates on case and aggregate review.
- Support signal detection and safety monitoring.
- Participate in client meetings, safety review discussions, and regulatory inspections.
Key Skills Across Roles:
- Strong understanding of pharmacovigilance regulations, ICSR processes, and GxP compliance.
- Proficiency in ARGUS Safety Database and PV systems.
- Excellent analytical, communication, and problem-solving skills.
- Attention to detail, accuracy, and speed in case processing.
- Ability to work collaboratively with cross-functional teams and clients.
Eligibility / Qualifications
- Educational Qualification: B.Pharm, M.Pharm, Pharm.D for ICSR/Data Entry/Quality Review roles; MBBS/MD (Pharmacology) for Drug Safety Physician.
- Experience:
- ICSR Book-In: 1–3 years
- Data Entry: 2–5 years
- Quality Review & Regulatory Report Submission: 4–6 years
- Drug Safety Physician: 0–2 years (post MD/MBBS)
- Preferred Courses: B.Pharm, M.Pharm, Pharm.D, MBBS, MD Pharmacology, Clinical Research, Drug Safety, Life Sciences.
Location & Work Type
- Location: Vizen Life Sciences, Kapil Towers, 10th Floor, Gachibowli, Hyderabad, India
- Work Mode: 100% On-Site
- Schedule: Alternate Saturdays working
Application Process
Advance your career in pharmacovigilance and drug safety operations:
📧 Apply via email: Careers@vizenlifesciences.com
🌐 Company Careers Page: Vizen Life Sciences Careers
Apply promptly to secure your opportunity in ICSR processing, regulatory submissions, and drug safety monitoring.
Why Join Vizen Life Sciences?
- Work with a leading pharmacovigilance and drug safety service provider.
- Gain hands-on experience in ICSR book-in, data entry, quality review, and regulatory reporting.
- Contribute to patient safety, regulatory compliance, and global drug safety initiatives.
- Collaborate with experienced PV professionals in a dynamic and structured environment.
- Build a career in high-demand, high CPC pharmacovigilance roles.
FAQs
Q1: Who can apply for these PV roles?
B.Pharm, M.Pharm, Pharm.D, MBBS/MD Pharmacology graduates with relevant PV experience.
Q2: What work mode is offered?
All roles are on-premise at Hyderabad.
Q3: What experience levels are required?
Roles range from 1–6 years, depending on position.
Q4: How to apply?
Send your resume to Careers@vizenlifesciences.com or apply via Vizen Careers.
Q5: Are alternate Saturdays working?
Yes, as per the work schedule.
| Company | Vizen Life Sciences Pvt Ltd |
|---|---|
| Vacancies | Full-Time, On-Site |
| Required Education | B.Pharm, M.Pharm, Pharm.D, MBBS, MD Pharmacology, Clinical Research, Drug Safety, Life Sciences |
| Experience | 1–6 years depending on role in PV, ICSR book-in, quality review, or medical review |
| Location | Hyderabad, India |
To apply for this job email your details to Careers@vizenlifesciences.com

