Corona Walk-in Production, QA, QC

- B.Pharm & ITI Openings at Corona Remedies – Ahmedabad
- Company Overview
- Job Role & Responsibilities
- Production – Senior Officer / Executive (B.Pharm)
- Production – Operator (ITI)
- Quality Assurance – Executive / Senior Officer (IPQA)
- Quality Control – Senior Officer / Executive (B.Pharm / M.Sc)
- Eligibility / Qualifications
- Technical Skills & Attributes Required
- Location & Salary
- Walk-in & Application Process
- FAQ
- Summary Table
B.Pharm & ITI Openings at Corona Remedies – Ahmedabad
Hiring B.Pharm, M.Sc, ITI professionals for Production, QA, QC roles. Multiple vacancies at Corona Remedies, Ahmedabad. Walk-in 30 Nov 2025.
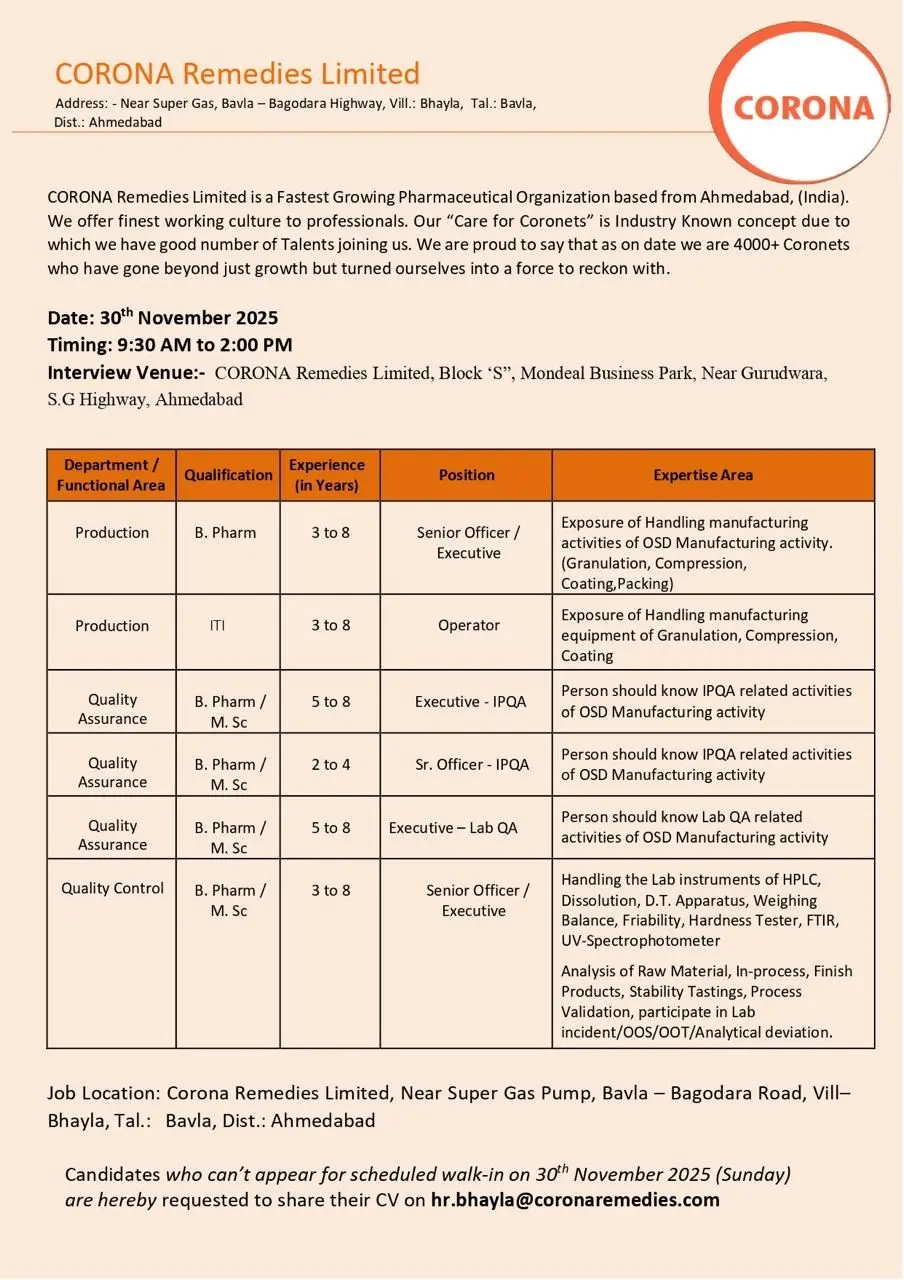
Corona Remedies Limited (Ahmedabad) invites experienced formulation professionals to a campus-style walk-in interview for multiple Production, Quality Assurance (IPQA), and Quality Control positions. If you have hands-on OSD manufacturing experience—granulation, compression, coating, packing—or lab expertise with HPLC and stability testing, this is an opportunity to join a fast-growing pharma company with a strong culture of employee care.
Company Overview
Corona Remedies Limited is a rapidly expanding pharmaceutical organisation headquartered in Ahmedabad. The company emphasizes a people-first culture dubbed “Care for Coronets,” and has grown to a workforce of 4,000+ employees. Corona focuses on oral solid dosage (OSD) manufacturing and maintains robust in-house quality processes to support consistent product performance and regulatory compliance.
Job Role & Responsibilities
Production – Senior Officer / Executive (B.Pharm)
- Manage end-to-end OSD manufacturing activities including granulation, compression, coating and packing.
- Supervise operators, maintain batch records, and ensure GMP adherence during production.
- Coordinate with QA for line clearances, sampling, and in-process controls.
- Troubleshoot production issues and support process validation activities.
Production – Operator (ITI)
- Operate granulation, compression and coating equipment under supervision.
- Execute routine preventive maintenance checks and report deviations.
- Ensure equipment hygiene and follow safety and GMP procedures.
Quality Assurance – Executive / Senior Officer (IPQA)
- Conduct IPQA activities: line clearance, in-process sampling, environmental monitoring, and batch record review.
- Support deviation investigations, OOS/OOT handling, and quality notifications.
- Collaborate with production and QC to ensure batch compliance and release readiness.
Quality Control – Senior Officer / Executive (B.Pharm / M.Sc)
- Perform analytical testing: HPLC, dissolution, UV, FTIR, friability, hardness, and weighing balance operations.
- Analyse raw material, in-process, finished product, and stability samples.
- Participate in lab investigations (OOS/OOT), method validation, and process validation support.
- Maintain accurate analytical documentation and ensure GLP/GMP-compliant data integrity.
Eligibility / Qualifications
- Production (Senior Officer/Executive): B.Pharm, 3–8 years experience in OSD manufacturing (granulation, compression, coating, packing).
- Production (Operator): ITI, 3–8 years experience operating OSD manufacturing equipment.
- QA – IPQA (Executive & Sr. Officer): B.Pharm/M.Sc, 2–8 years (role dependent) with IPQA exposure in OSD operations.
- QC – Senior Officer/Executive: B.Pharm/M.Sc, 3–8 years handling HPLC, dissolution apparatus, and stability testing.
Relevant courses (comma-separated): B.Pharm, M.Pharm, M.Sc Pharmaceutical Chemistry, M.Sc Analytical Chemistry, M.Sc Microbiology, ITI (Mechanical/Electrical/Instrumentation), PG Diploma in Quality Assurance, Certificate in GMP, Certificate in Sterile/OSD Manufacturing.
Technical Skills & Attributes Required
- Hands-on experience with HPLC, dissolution testing, FTIR, UV spectrophotometer, hardness & friability testers.
- Strong knowledge of GMP, documentation practices, and batch record management.
- Exposure to process validation, stability testing, and lab incident investigations (OOS/OOT).
- Good communication, team coordination, and problem-solving skills.
- Willingness to work in rotational shifts as per plant requirements.
Location & Salary
- Work Location: Corona Remedies Limited, Near Super Gas Pump, Bavla Bagodara Road, Vill‑Bhayla, Tal.: Bavla, Dist.: Ahmedabad.
- Walk-in Interview: 30th November 2025 (Sunday), 09:30 AM to 02:00 PM at Block “S”, Mondeal Business Park, Near Gurudwara, S.G. Highway, Ahmedabad.
- Salary: Competitive, based on experience and industry standards. Exact compensation will be discussed during the interview.
Walk-in & Application Process
- Walk-in Date: 30/11/2025 (Sunday)
- Time: 09:30 AM – 02:00 PM
- Venue: CORONA Remedies Limited, Block “S”, Mondeal Business Park, Near Gurudwara, S.G Highway, Ahmedabad.
- Contact / CV submission (if you cannot attend): hr.bhayla@coronaremedies.com (mailto:hr.bhayla@coronaremedies.com)
How to prepare: Bring updated CV, original and photo copies of educational and experience certificates, and any documents proving previous OSD manufacturing or analytical experience.
FAQ
Q: Can freshers apply?
A: No. These positions require 2–8 years of relevant experience as specified per role.
Q: Is formulation experience mandatory?
A: Yes. Only candidates with direct formulation/OSD manufacturing exposure will be considered for production and QA roles.
Q: What should I carry to the walk‑in interview?
A: Updated CV, original certificates, experience letters, and any audit/validation exposure documents.
Q: Can I send my CV if I miss the walk‑in date?
A: Yes. Send your resume to hr.bhayla@coronaremedies.com (mailto:hr.bhayla@coronaremedies.com).
Q: Are shifts rotational?
A: Yes. Candidates must be flexible to work in rotational shifts.
Summary Table
| Category | Details |
|---|---|
| Company | Corona Remedies Limited (Ahmedabad) |
| Vacancies | Production (Senior Officer/Executive, Operator), Quality Assurance (IPQA Executive/Sr. Officer), Quality Control (Senior Officer/Executive) |
| Required Education | B.Pharm, M.Sc, ITI, M.Pharm, PG Diploma in QA, Certificate in GMP |
| Experience | 2–8 years depending on role |