SP Accure Walk-in Engineering, HVAC, Instrumentation & Regulatory Affairs

- Company Overview
- Job Role & Responsibilities
- Engineering – HVAC & Water Systems
- Process Engineering (Injectable & OSD Equipment)
- Process Instrumentation (Injectable & OSD Equipment)
- Regulatory Affairs – Formulation Dossiers
- Regulatory Affairs – API (DMF/ASMF/CEP)
- Eligibility / Qualifications
- Location & Salary
- Application Process
- FAQs
- Is formulation experience mandatory?
- Are freshers eligible?
- Are female candidates eligible?
- What regulatory knowledge is required?
- Will there be shift work?
- Summary Table
BPharm MSc Regulatory & Engineering Roles – SP Accure Labs
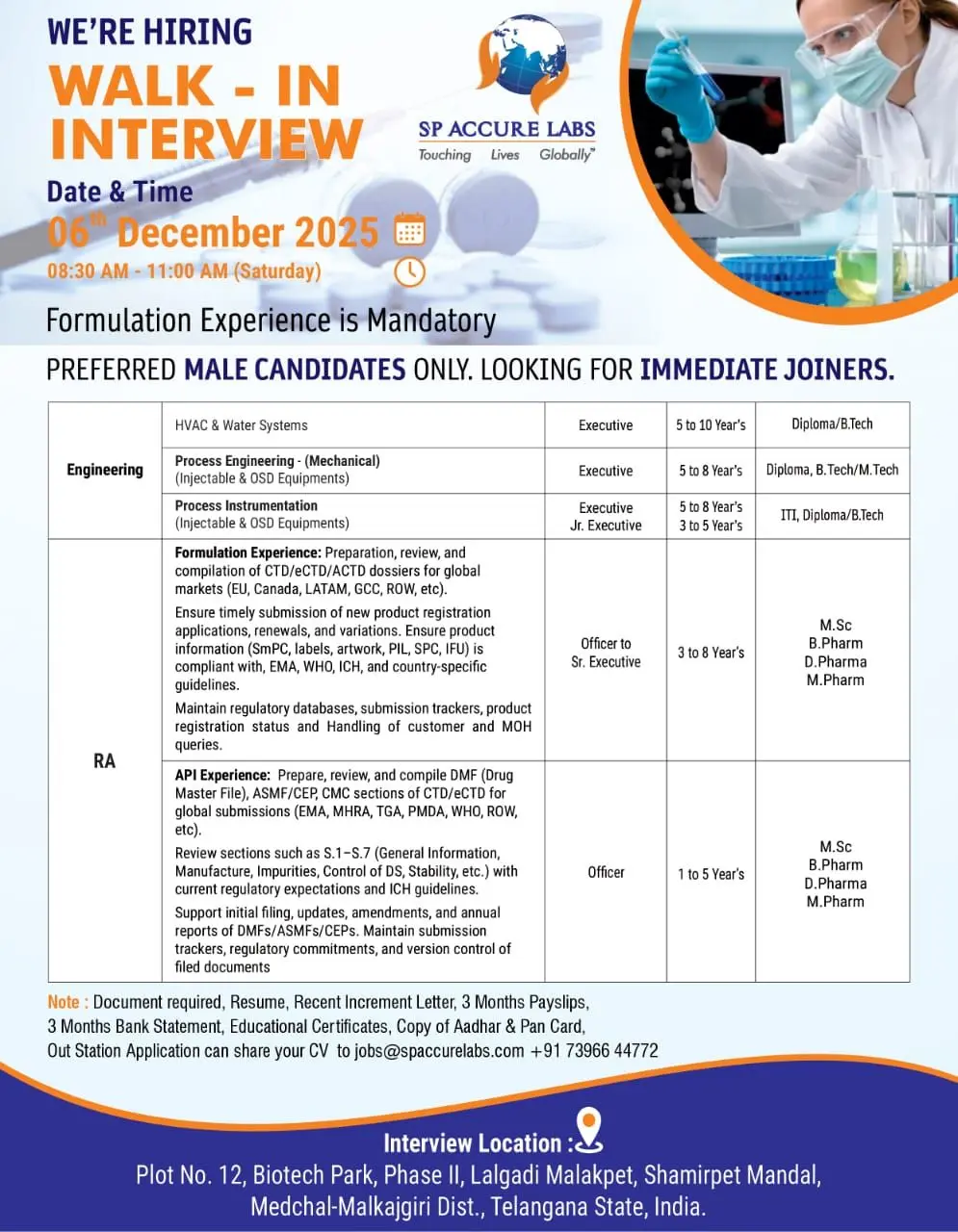
SP Accure Labs hiring for Engineering, HVAC, Instrumentation & Regulatory Affairs (3–10 yrs). Walk-in on 06 Dec 2025, Hyderabad.
SP Accure Labs is conducting a walk-in interview for multiple openings across Engineering, HVAC, Instrumentation and Regulatory Affairs. These roles are ideal for candidates with formulation or API experience and a strong understanding of CTD/eCTD submissions, DMF/ASMF preparation, and compliance with global regulatory frameworks. Immediate joiners and male candidates (as per job requirement) will be prioritized.
Company Overview
SP Accure Labs is a global pharmaceutical organization focused on high-quality formulations and regulatory-compliant manufacturing. Serving regulated markets such as the EU, Canada, LATAM, GCC and ROW, the company operates with robust QMS systems, modern engineering utilities, and a strong regulatory team. SP Accure is known for its dossier preparation expertise, regulatory compliance culture and end-to-end support for global product registrations.
Job Role & Responsibilities
Openings span Engineering, HVAC/Water Systems, Process Instrumentation, and Regulatory Affairs for Formulation and API dossiers.
Engineering – HVAC & Water Systems
Position: Executive
Qualification: Diploma / B.Tech
Experience: 3–8 years
Responsibilities:
- Manage and maintain HVAC operations for injectable and OSD facilities.
- Oversee WFI, purified water, and utility systems.
- Perform routine monitoring, calibration checks, and preventive maintenance.
- Ensure compliance with GMP, cGEP and cleanroom environmental standards.
Process Engineering (Injectable & OSD Equipment)
Position: Executive
Qualification: Diploma / B.Tech / M.Tech
Experience: 5–10 years
Responsibilities:
- Support engineering functions for OSD and Injectable equipment.
- Handle mechanical troubleshooting, process optimization and equipment qualification.
- Collaborate with production and maintenance teams for equipment readiness.
Process Instrumentation (Injectable & OSD Equipment)
Position: Jr. Executive / Executive
Qualification: ITI / Diploma / B.Tech
Experience: 5–8 years
Responsibilities:
- Maintain instrumentation panels, control systems and process sensors.
- Support calibration, troubleshooting and automation requirements.
- Ensure compliance with data integrity and audit expectations.
Regulatory Affairs – Formulation Dossiers
Position: Officer to Sr. Executive
Qualification: M.Sc / B.Pharm / D.Pharm / M.Pharm
Experience: 3–8 years
Responsibilities:
- Prepare, review and compile CTD/eCTD/ACTD dossiers for regulated markets.
- Ensure compliance with WHO, EMA, ICH and country-specific regulatory guidelines.
- Manage variations, renewals and new product submissions.
- Maintain regulatory databases, trackers and registration statuses.
- Handle customer and Ministry of Health (MOH) queries.
Regulatory Affairs – API (DMF/ASMF/CEP)
Position: Officer
Qualification: M.Sc / B.Pharm / D.Pharm / M.Pharm
Experience: 1–5 years
Responsibilities:
- Prepare, review and update DMFs, ASMFs/CEPs and CMC sections of CTD/eCTD.
- Manage S.1–S.7 sections per ICH guidelines.
- Support initial filings, updates, amendments, and annual reports.
- Maintain submission trackers, commitments and document version control.
Eligibility / Qualifications
Required Education: Diploma, B.Tech, M.Tech, ITI, M.Sc, B.Pharm, D.Pharm, M.Pharm.
Experience: 1–10 years depending on position.
Mandatory: Formulation experience for RA Formulations.
Preferred: Male candidates and immediate joiners.
Relevant Courses (comma-separated): Pharmaceutical Regulatory Affairs, Pharmaceutics, Industrial Pharmacy, Process Engineering, HVAC Operations, Instrumentation & Control, Mechanical Engineering, Analytical Sciences, CTD/eCTD Compilation, CMC Documentation.
Location & Salary
Interview Venue: Plot No. 12, Biotech Park Phase II, Lalgadi Malakpet, Shamirpet Mandal, Medchal-Malkajgiri, Telangana.
Date: 06 December 2025 (Saturday)
Time: 08:30 AM – 11:00 AM
Salary will be based on experience, regulatory expertise and engineering specialization.
Application Process
Bring the following documents:
- Updated Resume
- Recent Increment Letter
- 3 Months’ Pay Slips
- 3 Months’ Bank Statement
- Educational Certificates
- Aadhar & PAN copies
For out-station candidates: Email your CV to jobs@spaccurelabs.com or contact +91 73966 44772.
FAQs
Is formulation experience mandatory?
Yes, for RA Formulations roles. API RA roles require DMF/ASMF experience.
Are freshers eligible?
No. All openings require 1–10 years of experience.
Are female candidates eligible?
Roles prefer male candidates due to operational requirements.
What regulatory knowledge is required?
ICH, WHO, EMA guidelines; CTD/eCTD formats; CMC documentation; DMF/ASMF/CEP.
Will there be shift work?
Engineering and instrumentation roles may require shift flexibility.
Summary Table
| Category | Details |
|---|---|
| Company | SP Accure Labs |
| Vacancies | Engineering, HVAC, Instrumentation, RA Formulation, RA API |
| Required Education | Diploma, B.Tech, M.Tech, ITI, M.Sc, B.Pharm, D.Pharm, M.Pharm |
| Experience | 1–10 years depending on department |