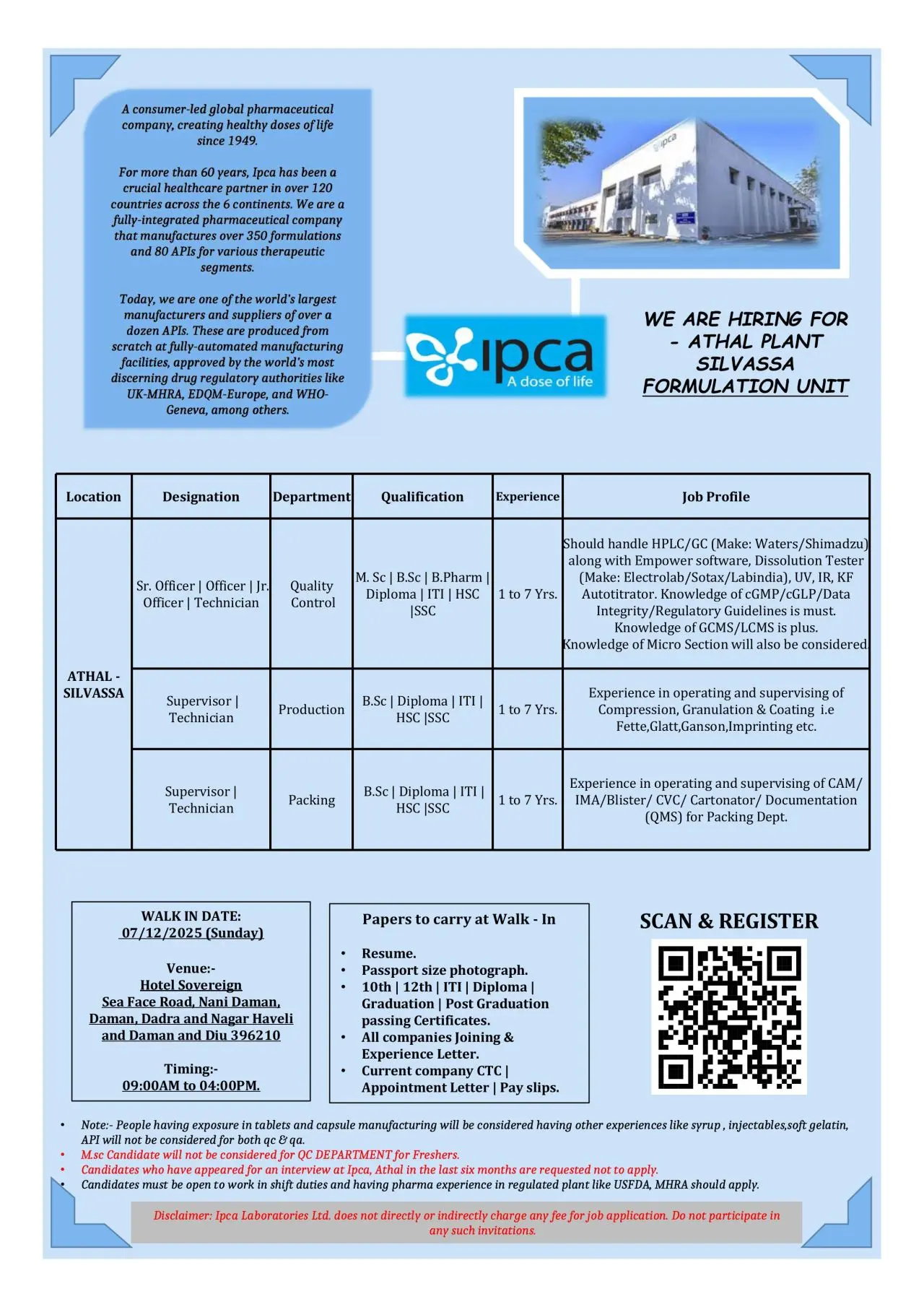
Ipca Walk-in QC Production Packing

- Company Overview
- Job Role & Responsibilities
- Quality Control – Sr. Officer / Officer / Jr. Officer / Technician
- Production – Supervisor / Technician
- Packing – Supervisor / Technician
- Eligibility / Qualifications
- Required Education
- Experience Requirements
- Location & Salary
- Walk-In Interview Details
- Application Process
- FAQs
- Who is eligible for these openings?
- Are freshers allowed?
- What experience is mandatory?
- Will candidates from API or injectable backgrounds be considered?
- Does Ipca charge fees for recruitment?
- Summary Table
Diploma ITI QC Production Packing Openings – Ipca Athal
Ipca Athal Silvassa hiring Diploma/ITI/BSc/BPharm candidates for QC, Production & Packing roles. Walk-in on 7 Dec 2025.
Ipca Laboratories is conducting a walk-in drive for multiple core functions at its Athal Formulation Unit in Silvassa. These openings are ideal for candidates experienced in tablet and capsule manufacturing within regulated environments and familiar with cGMP, documentation, and analytical systems.
Company Overview
Ipca Laboratories is a global, consumer-led pharmaceutical organization delivering quality medicines across 120+ countries. With more than six decades of operational excellence, Ipca manufactures over 350 formulations and 80 APIs at facilities approved by UK-MHRA, EDQM, WHO-Geneva and other major regulatory agencies. The Athal Unit specializes in high-compliance formulation manufacturing backed by automated systems, strong QMS, and data integrity standards.
Job Role & Responsibilities
Positions are open in Quality Control, Production and Packing.
Quality Control – Sr. Officer / Officer / Jr. Officer / Technician
Qualification: M.Sc, B.Sc, B.Pharm, HSC, SSC, Diploma, ITI
Experience: 1–7 years
Responsibilities:
- Operate analytical instruments such as HPLC, GC (Waters/Shimadzu), Dissolution Apparatus (Electrolab/Sotax/Labindia), UV, IR, KF Autotitrator.
- Manage QC documentation following cGMP, GLP and Data Integrity requirements.
- Assist in method execution, routine testing, stability studies and raw material/FP testing.
- Knowledge of GCMS/LCMS is an added advantage.
- Candidates with microbiology exposure will also be considered.
Important Note: M.Sc freshers will not be considered for QC.
Production – Supervisor / Technician
Qualification: B.Sc, Diploma, ITI, HSC, SSC
Experience: 1–7 years
Responsibilities:
- Operate and supervise granulation, compression and coating equipment.
- Experience with machines such as Fette, Glatt, Ganson, and imprinting systems.
- Maintain BMR/BPR, equipment logs, cleaning records and QMS documentation.
- Follow safety, cGMP and batch execution protocols.
Mandatory: Experience must be from tablets/capsules manufacturing. Exposure to syrups, injectables, soft-gel or API will not be considered.
Packing – Supervisor / Technician
Qualification: HSC, SSC
Experience: 1–7 years
Responsibilities:
- Operate CAM, IMA, Blister, CVC, Cartonator and inspection systems.
- Support QMS documentation for packing operations.
- Ensure packing quality checks, reconciliation and label verification.
- Adhere to cGMP and packing compliance standards.
Eligibility / Qualifications
Required Education
- SSC, HSC
- ITI, Diploma
- B.Sc
- M.Sc
- B.Pharm
Relevant Courses: Industrial Chemistry, Pharmaceutical Analysis, Pharmaceutics, Mechanical Operations, GMP & Quality Compliance.
Experience Requirements
- QC: 1–7 years
- Production: 1–7 years
- Packing: 1–7 years
Candidates must have prior pharma formulation experience in regulated plants (USFDA, MHRA).
Location & Salary
Walk-in Venue:
Hotel Sovereign, Sea Face Road, Nani Daman, Daman, Dadra & Nagar Haveli, Daman & Diu – 396210.
Salary will depend on skills, experience and functional expertise.
Walk-In Interview Details
- Date: 07 December 2025 (Sunday)
- Time: 09:00 AM – 04:00 PM
Candidates must be open to shift duties.
Application Process
Carry the following documents:
- Updated resume
- Passport-size photograph
- 10th/12th/ITI/Diploma/Degree certificates
- Joining & experience letters from all previous companies
- Appointment letter with CTC details
- Latest pay slips and 3 months bank statement
Candidates who appeared for interviews at Ipca Athal within the last 6 months should not apply.
Registration link available via QR code on official flyer.
FAQs
Who is eligible for these openings?
Candidates with 1–7 years of experience in tablets/capsules formulation units.
Are freshers allowed?
M.Sc freshers are not eligible for QC. All departments require prior formulation experience.
What experience is mandatory?
Tablet/capsule manufacturing experience in regulated plants (USFDA/MHRA).
Will candidates from API or injectable backgrounds be considered?
No. Only tablet/capsule formulation experience will be accepted.
Does Ipca charge fees for recruitment?
No. Ipca does not charge any application or recruitment fee.
Summary Table
| Category | Details |
|---|---|
| Company | Ipca Laboratories Ltd – Athal Formulation Unit |
| Vacancies | QC, Production, Packing (Technician to Sr. Officer) |
| Required Education | SSC, HSC, ITI, Diploma, B.Sc, M.Sc, B.Pharm |
| Experience | 1–7 years in tablet/capsule formulation units |