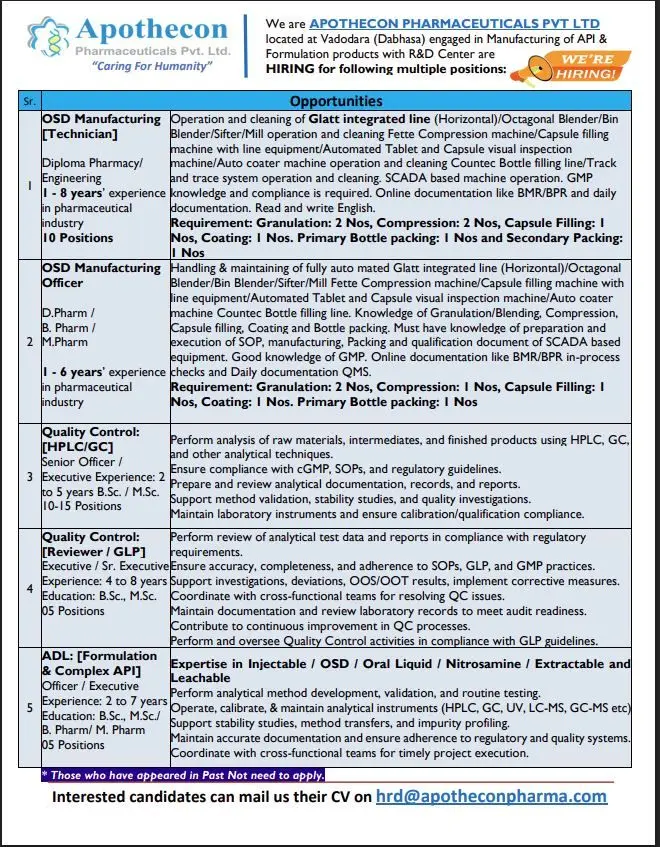
Apothecon Hiring OSD Manufacturing, QC, and ADL

- Company Overview
- Job Role & Responsibilities
- 1. OSD Manufacturing [Technician]
- 2. OSD Manufacturing [Officer]
- 3. Quality Control [HPLC/GC]
- 4. Quality Control [Reviewer / GLP]
- 5. Analytical Development Laboratory (ADL) – Formulation & Complex API
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join Apothecon Pharmaceuticals?
- FAQs
- Summary Table
B.Pharm, M.Pharm, Diploma Openings at Apothecon Pharmaceuticals – Vadodara
Apothecon Pharmaceuticals Vadodara hiring B.Pharm, M.Pharm, Diploma candidates for OSD Manufacturing, QC, and ADL departments. Multiple vacancies open.
Apothecon Pharmaceuticals Pvt. Ltd., a leading API and formulation manufacturer located in Vadodara (Dabhasa), is inviting skilled and passionate pharma professionals to join its growing team. With a strong R&D foundation and a commitment to quality, the company continues to advance healthcare solutions through innovation and excellence. If you are looking to build a meaningful career in a regulated pharma environment, this is your opportunity to work with a globally trusted brand.
Company Overview
Apothecon Pharmaceuticals Pvt. Ltd. is a well-established pharmaceutical manufacturer known for its high-quality API and formulation products. The company operates a state-of-the-art facility in Vadodara (Dabhasa) and is driven by the mission of Caring for Humanity. Its R&D Center supports continuous product innovation and regulatory compliance across global markets. Apothecon upholds strong values of integrity, quality, and sustainability in all its operations.
Job Role & Responsibilities
1. OSD Manufacturing [Technician]
- Qualification: Diploma in Pharmacy / Engineering
- Experience: 1 to 8 years in the pharmaceutical industry
- Vacancies: 10 positions
Key Responsibilities:
- Operate and clean equipment like Glatt Integrated Line (Horizontal), Octagonal Blender, Bin Blender, Sifter, Mill.
- Manage operations for Fette Compression machine, Capsule filling, Auto Coater, and Visual Inspection machines.
- Handle Countec Bottle filling line and Track & Trace systems.
- Perform online documentation (BMR/BPR) and maintain GMP compliance.
- Understand SCADA-based machine operation and ensure adherence to quality standards.
Departmental Openings:
- Granulation: 2 Nos
- Compression: 2 Nos
- Capsule Filling: 1 No
- Coating: 1 No
- Primary Bottle Packing: 1 No
- Secondary Packing: 1 No
2. OSD Manufacturing [Officer]
- Qualification: D.Pharm / B.Pharm / M.Pharm
- Experience: 1 to 6 years
- Vacancies: Multiple
Responsibilities:
- Manage and maintain fully automated Glatt integrated lines and related equipment.
- Knowledge of Granulation, Blending, Compression, Capsule Filling, Coating, and Bottle Packing.
- Prepare and execute SOPs, manufacturing, packing, and qualification documentation.
- Strong understanding of QMS, GMP compliance, and online documentation (BMR/BPR).
Departmental Requirements:
- Granulation: 2 Nos
- Compression: 1 No
- Capsule Filling: 1 No
- Coating: 1 No
- Primary Bottle Packing: 1 No
3. Quality Control [HPLC/GC]
- Designation: Senior Officer / Executive
- Qualification: B.Sc. / M.Sc.
- Experience: 2 to 5 years
- Vacancies: 10–15 positions
Responsibilities:
- Analyze raw materials, intermediates, and finished products using HPLC, GC, and other analytical instruments.
- Ensure cGMP and SOP compliance.
- Prepare and review analytical reports, method validation, and stability documentation.
- Maintain lab instruments and perform calibration/qualification.
- Support investigations and quality improvements.
4. Quality Control [Reviewer / GLP]
- Designation: Executive / Sr. Executive
- Qualification: B.Sc. / M.Sc.
- Experience: 4 to 8 years
- Vacancies: 5 positions
Responsibilities:
- Review analytical data, reports, and ensure GLP/GMP adherence.
- Investigate deviations, OOS/OOT results, and propose corrective actions.
- Collaborate with cross-functional teams for quality issue resolution.
- Maintain audit-ready documentation and contribute to process optimization.
5. Analytical Development Laboratory (ADL) – Formulation & Complex API
- Designation: Officer / Executive
- Qualification: B.Sc., M.Sc., B.Pharm, M.Pharm
- Experience: 2 to 7 years
- Vacancies: 5 positions
Responsibilities:
- Perform analytical method development, validation, and stability studies.
- Handle analytical instruments such as HPLC, GC, UV, LC-MS, GC-MS.
- Conduct impurity profiling and method transfers.
- Maintain regulatory-compliant documentation.
- Coordinate with R&D and manufacturing teams for timely project execution.
Eligibility / Qualifications
- B.Pharm, M.Pharm, D.Pharm, B.Sc., M.Sc., or Engineering background
- Strong knowledge of GMP, QMS, and SCADA-based systems
- Excellent communication skills in English
- Prior experience in OSD manufacturing, QC, or ADL preferred
Location & Salary
Location: Vadodara (Dabhasa), Gujarat
Salary: As per industry standards and experience level
Application Process
Interested and eligible candidates can send their updated CVs to hrd@apotheconpharma.com.
Note: Candidates who have appeared for interviews in the past are requested not to reapply.
Apply before November 10, 2025, to secure your interview slot.
Why Join Apothecon Pharmaceuticals?
- Opportunity to work with advanced pharmaceutical manufacturing systems.
- Exposure to global regulatory standards and audits.
- Work in a collaborative, growth-oriented environment.
- Gain hands-on experience in API and formulation production.
FAQs
1. What qualifications are required to apply?
Candidates must hold a D.Pharm, B.Pharm, M.Pharm, B.Sc., M.Sc., or Diploma in Engineering from a recognized institution.
2. Is prior experience mandatory?
Yes, depending on the role. Technicians and Officers require 1–8 years of experience in the pharmaceutical manufacturing sector.
3. Where is the job location?
All positions are based at the Vadodara (Dabhasa) plant in Gujarat.
4. What is the selection process?
Shortlisted candidates will be contacted by HR for an interview based on their qualification and experience.
5. When is the last date to apply?
Applications are accepted until November 10, 2025.
Summary Table
| Category | Details |
|---|---|
| Company | Apothecon Pharmaceuticals Pvt. Ltd. |
| Vacancies | Multiple (Technician, Officer, QC, ADL) |
| Required Education | D.Pharm, B.Pharm, M.Pharm, B.Sc., M.Sc., Engineering |
| Experience | 1–8 years |
| Location | Vadodara (Dabhasa), Gujarat |
| Email to Apply | hrd@apotheconpharma.com |
To apply for this job email your details to hrd@apotheconpharma.com


