Indoco Walk‑In Production, QA, QC, Engineering

- Company Overview
- Job Role & Responsibilities
- Production – OSD (Tablet Manufacturing & Packing)
- Quality Assurance – IPQA
- Quality Assurance – Process Validation
- Quality Control – Stability In‑Charge
- Quality Control – Finished Goods (FG) In‑Charge
- Engineering – QMS / HVAC
- Engineering – Utility / Process Maintenance (Electrical)
- Eligibility / Qualifications
- Educational Qualifications
- Experience Requirements
- Location & Salary
- Walk‑In Interview Details
- Documents to Carry
- Application Process
- Why Join Indoco Remedies?
- Frequently Asked Questions (FAQs)
- Is this walk‑in open to freshers?
- Is shift work required?
- What regulatory exposure will candidates gain?
BPharm/MSc Pharma Vacancies Indoco Baddi Walk‑In
Indoco Remedies hiring Production, QA, QC, Engineering professionals at Baddi. BPharm/MPharm/MSc with 2–12 years experience.
Pharmaceutical manufacturing relies on strong production systems, rigorous quality oversight, and dependable engineering support to ensure safe and effective medicines reach patients. Indoco Remedies Limited is conducting a walk‑in interview at its Unit‑III facility in Baddi, Himachal Pradesh, to strengthen its Oral Solid Dosage (OSD) operations. This opportunity is aimed at experienced professionals across Production, Quality Assurance, Quality Control, and Engineering who are looking to grow their careers in a regulated, compliance‑driven pharmaceutical organization.
Company Overview
Indoco Remedies Limited is a well‑established Indian pharmaceutical company with a strong presence in domestic and international markets. The company manufactures and supplies high‑quality finished dosage formulations and APIs, with facilities approved by leading regulatory authorities. Indoco’s operations are guided by stringent cGMP standards, robust quality systems, and a strong commitment to patient safety.
The Baddi manufacturing facility plays a key role in Indoco’s formulation business, particularly in Oral Solid Dosage manufacturing. Professionals working at this site gain exposure to regulated manufacturing environments, cross‑functional collaboration, and continuous improvement initiatives aligned with global pharmaceutical standards.
Job Role & Responsibilities
This walk‑in drive covers multiple critical departments. Selected candidates will contribute directly to compliant pharmaceutical manufacturing and quality systems.
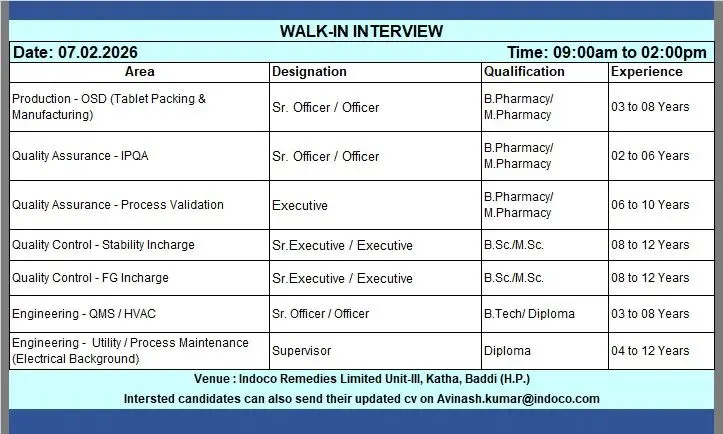
Production – OSD (Tablet Manufacturing & Packing)
- Execute tablet manufacturing and packing operations as per SOPs and BMRs
- Ensure compliance with GMP, safety, and data integrity requirements
- Monitor in‑process controls and maintain accurate production documentation
- Support troubleshooting and productivity improvement initiatives
Quality Assurance – IPQA
- Perform in‑process quality checks during manufacturing and packing
- Review batch manufacturing and packing records
- Handle deviations, investigations, and CAPA documentation
- Ensure compliance with cGMP and regulatory expectations
Quality Assurance – Process Validation
- Plan and execute process validation activities
- Review validation protocols, reports, and related documentation
- Support regulatory audits and validation lifecycle management
- Ensure validation compliance across manufacturing processes
Quality Control – Stability In‑Charge
- Manage stability studies as per approved protocols
- Oversee stability chamber operation, sample management, and data trending
- Ensure compliance with regulatory guidelines and documentation practices
Quality Control – Finished Goods (FG) In‑Charge
- Supervise testing and release of finished goods
- Review analytical data and ensure GLP compliance
- Coordinate with QA for batch disposition and investigations
Engineering – QMS / HVAC
- Maintain HVAC systems and engineering QMS documentation
- Support qualification, calibration, and compliance activities
- Ensure engineering systems meet GMP and safety standards
Engineering – Utility / Process Maintenance (Electrical)
- Handle preventive and breakdown maintenance of utilities and process equipment
- Ensure electrical systems operate reliably and safely
- Support continuous improvement and compliance initiatives
Eligibility / Qualifications
Educational Qualifications
- Production / QA: B.Pharmacy, M.Pharmacy
- Quality Control: B.Sc, M.Sc
- Engineering: B.Tech, Diploma (Electrical/Mechanical)
Relevant courses include: B.Pharm, M.Pharm, B.Sc Chemistry, M.Sc Chemistry, Diploma in Electrical Engineering, Diploma in Mechanical Engineering, B.Tech Electrical, B.Tech Mechanical
Experience Requirements
- Production (OSD): 3 to 8 years
- QA – IPQA: 2 to 6 years
- QA – Process Validation: 6 to 10 years
- QC – Stability / FG: 8 to 12 years
- Engineering – QMS / HVAC: 3 to 8 years
- Engineering – Utility (Electrical): 4 to 12 years
Location & Salary
- Job Location: Indoco Remedies Limited, Unit‑III, Katha, Baddi (Himachal Pradesh)
- Salary: Best in industry, based on experience and role
- Work Mode: Full‑time, on‑site
Walk‑In Interview Details
- Date: 07 February 2026
- Time: 09:00 AM to 02:00 PM
- Venue: Indoco Remedies Limited, Unit‑III, Katha, Baddi (H.P.)
Documents to Carry
- Updated resume
- Academic certificates
- Experience documents and pay slips
- Government ID proof
Application Process
Candidates unable to attend the walk‑in interview may apply by email.
- Email: Avinash.kumar@indoco.com
- Subject Line: Application – Department Name (Baddi)
Why Join Indoco Remedies?
- Opportunity to work with a reputed pharmaceutical manufacturer
- Exposure to regulated OSD manufacturing and quality systems
- Strong focus on GMP, validation, and compliance
- Long‑term career growth across Production, QA, QC, and Engineering
Frequently Asked Questions (FAQs)
Is this walk‑in open to freshers?
No. All positions require prior pharmaceutical industry experience.
Is shift work required?
Yes. Selected candidates must be willing to work in shifts as per operational needs.
What regulatory exposure will candidates gain?
Candidates will work in a cGMP‑compliant facility aligned with global regulatory standards.
| Company | Indoco Remedies Limited |
|---|---|
| Vacancies | Production OSD, QA IPQA, QA Validation, QC Stability & FG, Engineering |
| Required Education | B.Pharm, M.Pharm, B.Sc, M.Sc, B.Tech, Diploma |
| Experience | 2–12 Years (role dependent) |
To apply for this job email your details to Avinash.kumar@indoco.com


