Ipca Walk-in QA, QC & Production (API)

- Company Overview
- Job Role & Responsibilities
- Quality Assurance (API) – Sr. Chemist / Chemist
- APQR – Annual Product Quality Review
- Production (API) / IPQA – Sr. Officer / Officer
- Quality Control (API) – Sr. Officer / Officer
- Eligibility / Qualifications
- Required Education
- Experience Requirements
- Preferred Exposure
- Location & Salary
- Walk-In Interview Details
- Application Process
- FAQs
- Are freshers eligible?
- What regulatory exposure is preferred?
- Does this role involve shift work?
- What analytical instruments should QC candidates know?
- What is APQR experience used for?
- Summary Table
BSc MSc API QA QC Production Openings – Ipca Indore
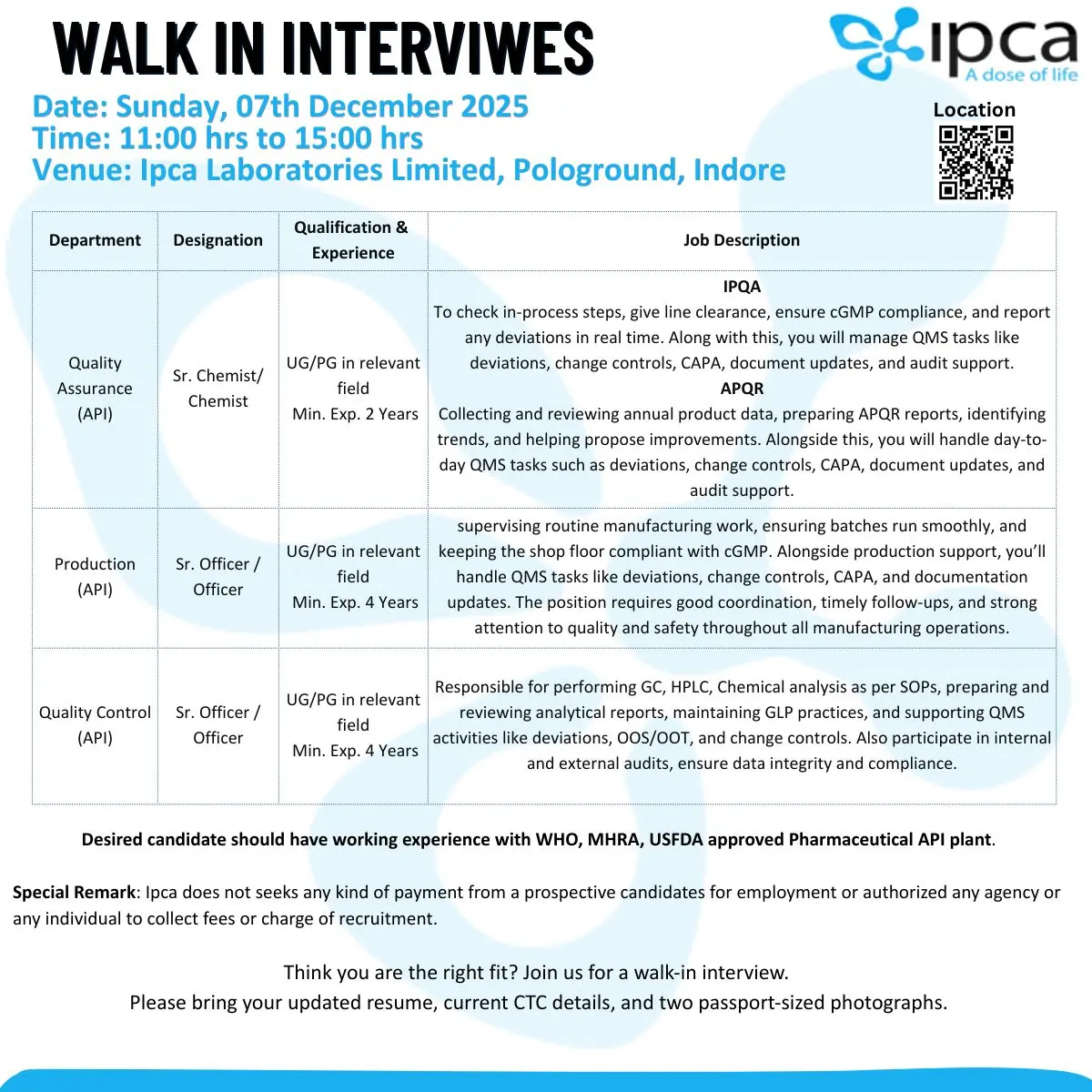
Ipca Indore hiring BSc/MSc candidates for QA, QC & Production (API) roles with 2–4+ yrs experience. Walk-in on 7 Dec 2025.
Ipca Laboratories is conducting walk-in interviews for multiple API functions including Quality Assurance, APQR, IPQA, Production, and Quality Control. These roles suit candidates with hands-on experience in regulated API manufacturing, strong QMS exposure, and practical understanding of cGMP systems.
Company Overview
Ipca Laboratories is a reputable global pharmaceutical manufacturer with strong API and formulation capabilities. The company holds approvals from WHO, MHRA, USFDA and several global agencies. Its Indore facility supports large-scale API operations with a focus on compliance, robust QMS, and continual improvement. Joining Ipca provides exposure to world-class manufacturing systems and regulatory-driven standards.
Job Role & Responsibilities
Openings span across QA, APQR, Production (API/IPQA), and QC.
Quality Assurance (API) – Sr. Chemist / Chemist
Qualification: UG/PG in relevant science discipline
Experience: Minimum 2 years
Responsibilities:
- Check in-process stages, ensure cGMP practices, and provide line clearance.
- Identify deviations in real time and escalate promptly.
- Manage QMS workflows including deviations, CAPA, change control, document revisions.
- Support plant audits and maintain compliance documentation.
APQR – Annual Product Quality Review
Qualification: UG/PG in relevant science discipline
Responsibilities:
- Collect, review, and compile annual product data.
- Prepare APQR reports and identify key trends.
- Support improvement proposals based on data observations.
- Handle QMS elements including deviations, CAPA, change controls, and audit support.
Production (API) / IPQA – Sr. Officer / Officer
Qualification: UG/PG in relevant discipline
Experience: Minimum 4 years
Responsibilities:
- Supervise batch operations and ensure smooth manufacturing.
- Maintain cGMP compliance at shop floor level.
- Support QMS activities: deviations, CAPA, change controls, documentation updates.
- Coordinate between production, QA, and maintenance to maintain operational discipline.
- Follow safety protocols, process monitoring, and timely escalation.
Quality Control (API) – Sr. Officer / Officer
Qualification: UG/PG in relevant science discipline
Experience: Minimum 4 years
Responsibilities:
- Perform GC, HPLC, and chemical analysis as per SOPs.
- Prepare and review analytical reports and maintain GLP records.
- Support QMS activities including deviations, OOS/OOT, change controls.
- Participate in internal/external audits and maintain data integrity.
- Ensure full compliance with WHO/MHRA/USFDA expectations.
Eligibility / Qualifications
Required Education
- B.Sc / M.Sc (Chemistry / Life Sciences)
- Other UG/PG science disciplines relevant to API manufacturing
Relevant courses: Analytical Chemistry, Industrial Chemistry, Organic Chemistry, Pharmaceutical Chemistry, API Technology.
Experience Requirements
- QA / APQR: Minimum 2 years
- Production / IPQA: Minimum 4 years
- QC: Minimum 4 years
Preferred Exposure
- Experience in WHO, MHRA, USFDA approved API manufacturing plants.
- Strong documentation ability and QMS competence.
Location & Salary
Walk-in Venue: Ipca Laboratories Ltd., Pologround, Indore
Job Location: API Manufacturing Facility, Indore
Salary will be offered based on role seniority, experience, and technical capabilities.
Walk-In Interview Details
- Date: 07 December 2025 (Sunday)
- Time: 11:00 AM – 03:00 PM
- Venue: Ipca Laboratories Limited, Pologround, Indore
Application Process
Walk-in directly with:
- Updated CV
- Current CTC details
- Two passport-size photographs
- Educational & experience certificates
Ipca does not charge any fees for recruitment and has not authorized any external agency to do so.
FAQs
Are freshers eligible?
No. Minimum required experience must be met.
What regulatory exposure is preferred?
WHO, MHRA, USFDA exposure is strongly preferred.
Does this role involve shift work?
Production and QC roles may require shift operations.
What analytical instruments should QC candidates know?
GC, HPLC, Ion Chromatography, wet chemistry techniques.
What is APQR experience used for?
Annual product review, trend analysis, and continuous improvement initiatives.
Summary Table
| Category | Details |
|---|---|
| Company | Ipca Laboratories Limited |
| Vacancies | QA, APQR, IPQA/Production, QC |
| Required Education | B.Sc / M.Sc (Chemistry/Life Sciences) |
| Experience | 2–4+ years depending on role |