Ipca walk-in API Production, QC, QA & EHS

- Company Overview
- Walk-In Interview Details
- Job Role & Responsibilities
- Production – API
- Quality Control – API
- Quality Assurance & Lab QA – Formulation
- Engineering – API
- EHS – Environment, Health & Safety
- Trainee Positions – Freshers
- Eligibility / Qualifications
- Eligible Courses (comma-separated)
- Preferred Candidate Profile
- Documents Required
- Why Build Your Career at Ipca Laboratories
- Frequently Asked Questions (FAQs)
- Who can attend this walk-in interview?
- Is USFDA plant experience mandatory?
- Can I apply by email instead of attending?
- Are freshers eligible?
- Summary Table
BSc BPharm API Walk-In | Ipca Ratlam MP
Ipca Laboratories walk-in for API Production, QC, QA & EHS in Ratlam (MP). BSc, BPharm, BE/BTech, Diploma. 0–10 yrs.
Ipca Laboratories Limited is conducting a walk-in interview for multiple positions at its Ratlam, Madhya Pradesh facility. The company is hiring experienced professionals and fresh graduates across API Production, Quality Control, Quality Assurance, Engineering, and EHS departments. Candidates with exposure to WHO-GMP, MHRA, or USFDA-approved pharmaceutical plants are strongly encouraged to attend.
This is an excellent opportunity for professionals seeking API manufacturing jobs, QC chemist roles, QA officer vacancies, pharma engineering careers, or EHS officer positions in a globally compliant pharmaceutical environment.
Company Overview
Ipca Laboratories Limited is a fully integrated Indian pharmaceutical company with strong export operations and global regulatory approvals. The company manufactures both Active Pharmaceutical Ingredients (API) and finished formulations at world-class facilities approved by leading drug regulatory authorities including the USFDA.
Ipca is known for its robust quality systems, GMP-compliant production practices, and strong regulatory track record. The Ratlam unit plays a key role in API manufacturing and export-driven production, offering professionals exposure to regulated markets and audit-intensive operations.
Working at Ipca provides hands-on experience in batch manufacturing, advanced analytical testing, validation systems, regulatory documentation, and pharmaceutical plant maintenance under strict compliance frameworks.
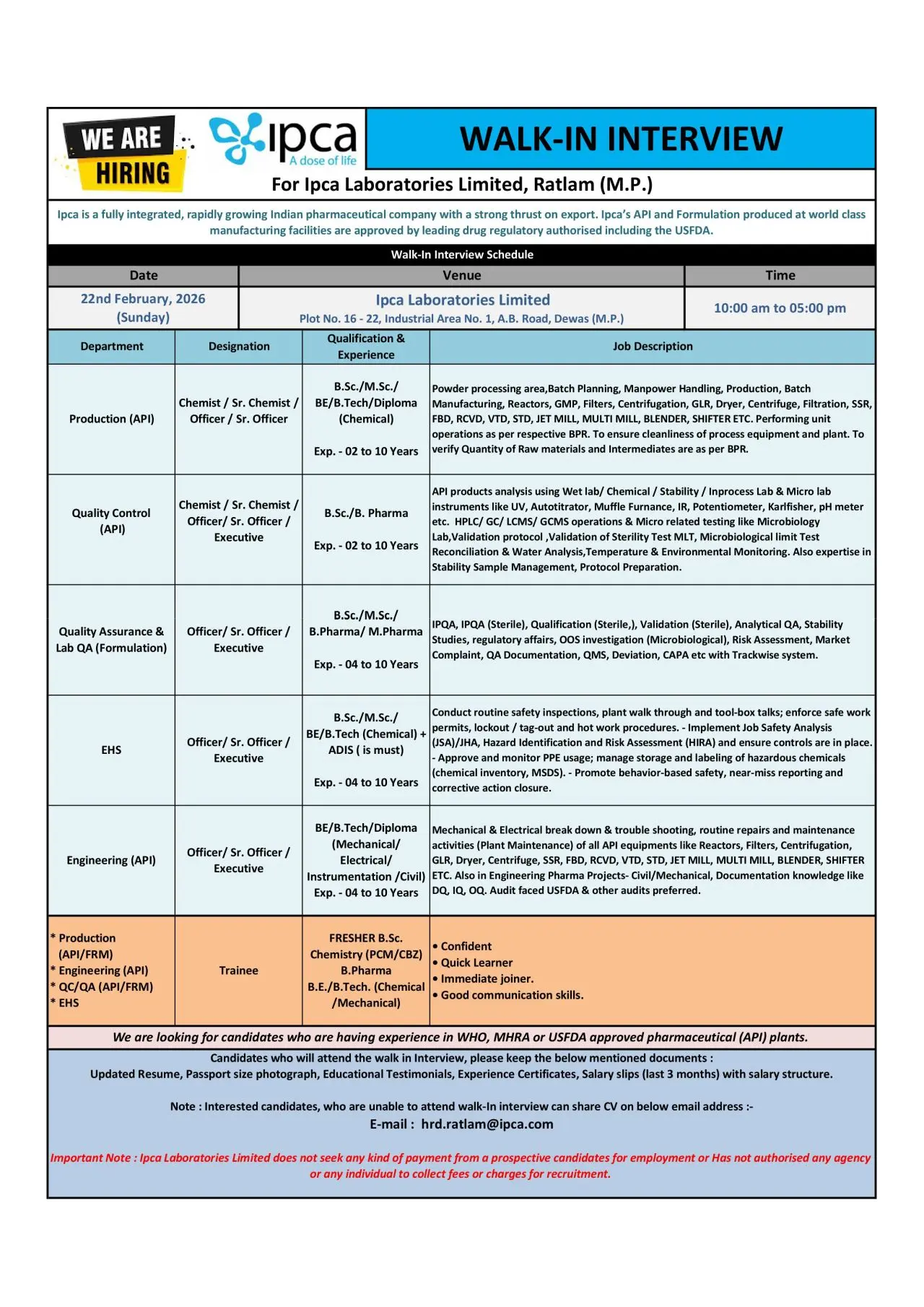
Walk-In Interview Details
Date: 22nd February 2026 (Sunday)
Time: 10:00 AM to 05:00 PM
Venue: Ipca Laboratories Limited, Plot No. 16–22, Industrial Area No. 1, A.B. Road, Dewas, Madhya Pradesh
Candidates unable to attend may share their updated CV at: hrd.ratlam@ipca.com
Job Role & Responsibilities
Production – API
Designation: Chemist / Sr. Chemist / Officer / Sr. Officer
Qualification: B.Sc, M.Sc, BE, B.Tech, Diploma (Chemical)
Experience: 2 to 10 Years
Key Responsibilities:
- Execute batch manufacturing and powder processing operations
- Operate reactors, GLR, centrifuges, dryers, filters, SSR, FBD, RCVD, VTD, STD, Jet Mill, Multi Mill, Blender, and Shifter
- Follow approved Batch Production Records (BPR)
- Ensure GMP compliance and equipment cleanliness
- Verify raw materials and intermediates as per BPR
- Handle manpower planning and shift coordination
Quality Control – API
Designation: Chemist / Sr. Chemist / Officer / Executive
Qualification: B.Sc, B.Pharm
Experience: 2 to 10 Years
Key Responsibilities:
- Perform API analysis using wet lab, chemical, stability, and in-process testing
- Operate analytical instruments including HPLC, GC, LCMS, GCMS, UV, IR, Karl Fischer, Autotitrator, Potentiometer, Muffle Furnace, and pH meter
- Conduct microbiological testing such as sterility testing, MLT, environmental monitoring, and water analysis
- Manage stability samples and prepare analytical protocols
- Maintain GLP documentation and ensure audit readiness
Quality Assurance & Lab QA – Formulation
Designation: Officer / Sr. Officer / Executive
Qualification: B.Sc, M.Sc, B.Pharm, M.Pharm
Experience: 4 to 10 Years
Key Responsibilities:
- Manage IPQA activities including sterile and non-sterile areas
- Handle validation and qualification activities
- Conduct OOS investigations and risk assessments
- Manage QMS elements such as deviation, CAPA, change control, and Trackwise documentation
- Support regulatory inspections and compliance audits
Engineering – API
Designation: Officer / Sr. Officer / Executive
Qualification: BE/B.Tech/Diploma (Mechanical, Electrical, Instrumentation, Civil)
Experience: 4 to 10 Years
Key Responsibilities:
- Troubleshoot and maintain API plant equipment
- Handle preventive and breakdown maintenance of reactors, centrifuges, dryers, mills, and filtration systems
- Support engineering documentation including DQ, IQ, OQ
- Participate in regulatory audits and compliance inspections
EHS – Environment, Health & Safety
Designation: Officer / Sr. Officer / Executive
Qualification: B.Sc/M.Sc/BE/B.Tech (Chemical) + ADIS (Mandatory)
Experience: 4 to 10 Years
Key Responsibilities:
- Conduct routine safety inspections and plant walkthroughs
- Implement lockout/tagout, hot work permits, and safe work systems
- Perform Hazard Identification and Risk Assessment (HIRA) and Job Safety Analysis (JSA)
- Manage hazardous chemical storage and MSDS documentation
- Promote behavior-based safety and near-miss reporting
Trainee Positions – Freshers
Qualification: B.Sc Chemistry (PCM/CBZ), B.Pharm, BE/B.Tech (Chemical/Mechanical)
Experience: Fresher
Fresh candidates will receive structured training in production, QC, QA, engineering, or EHS based on qualification and business needs.
Eligibility / Qualifications
Eligible Courses (comma-separated)
B.Sc Chemistry, M.Sc Chemistry, B.Pharm, M.Pharm, BE Chemical Engineering, B.Tech Chemical Engineering, BE Mechanical Engineering, B.Tech Mechanical Engineering, Diploma Chemical, Diploma Mechanical, ADIS
Candidates must demonstrate good communication skills, documentation discipline, and readiness to work in regulated API manufacturing environments.
Preferred Candidate Profile
- Experience in WHO, MHRA, or USFDA-approved API plants
- Knowledge of GMP, GLP, QMS, validation, and audit systems
- Immediate joiners preferred
- Confident and quick learners
Documents Required
Candidates attending the walk-in must carry:
- Updated resume
- Passport-size photograph
- Educational certificates
- Experience certificates
- Last 3 months salary slips with salary structure
Why Build Your Career at Ipca Laboratories
- Exposure to USFDA-approved API manufacturing
- Hands-on experience with advanced process equipment
- Strong regulatory and compliance culture
- Career progression in production, quality, and engineering domains
- Opportunities in export-focused pharmaceutical operations
These roles are ideal for candidates searching for pharmaceutical walk-in interviews, API production chemist jobs, QC analyst vacancies, QA officer pharma jobs, EHS officer careers, and GMP-compliant engineering positions.
Frequently Asked Questions (FAQs)
Who can attend this walk-in interview?
Candidates with relevant pharma experience or freshers meeting qualification criteria can attend.
Is USFDA plant experience mandatory?
It is preferred but not mandatory. Candidates from WHO/MHRA plants are also encouraged.
Can I apply by email instead of attending?
Yes. Share your CV at hrd.ratlam@ipca.com.
Are freshers eligible?
Yes. Trainee roles are available for eligible graduates.
Summary Table
| Company | Ipca Laboratories Limited |
|---|---|
| Vacancies | Production Chemist, QC Chemist, QA Officer, Engineering Executive, EHS Officer, Trainee |
| Required Education | B.Sc, M.Sc, B.Pharm, M.Pharm, BE, B.Tech, Diploma, ADIS |
| Experience | Fresher to 10 Years (Regulated API Preferred) |
To apply for this job email your details to hrd.ratlam@ipca.com


