V-Ensure Pharma Technologies Walk-In | QA/QC/Production

- Company Overview
- Job Roles & Responsibilities
- Quality Assurance (QA)
- Validation / IPQA
- Quality Control Analytical Lab
- Warehouse
- Production / Manufacturing
- Walk-In Drive Details
- Key Skills & Experience Required
- Benefits & Growth Opportunities
- FAQs
- V-Ensure Pharma Technologies Walkin
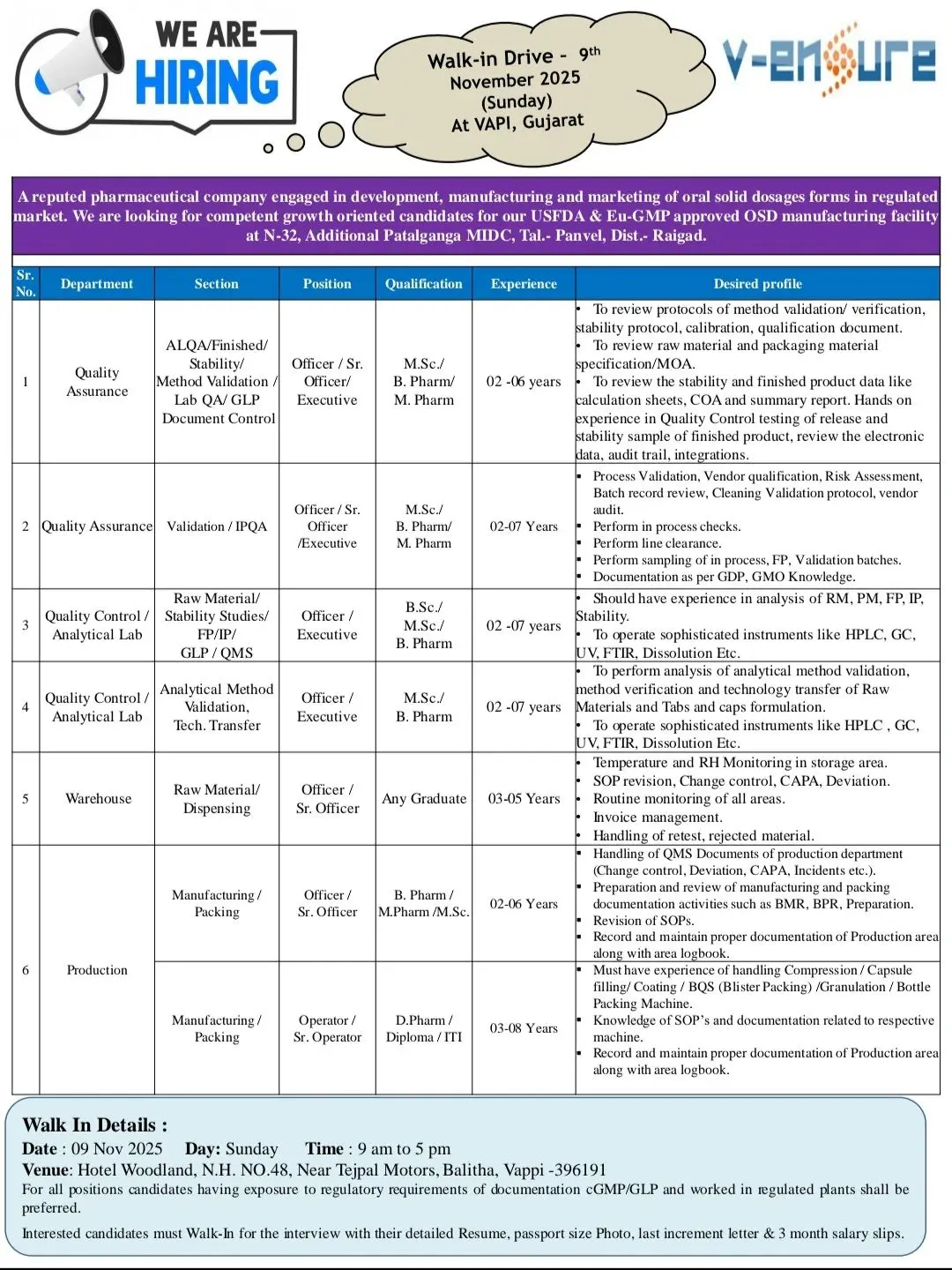
career with V-Ensure Pharma Technologies Pvt. Ltd., a reputed pharmaceutical company specializing in oral solid dosage manufacturing for regulated markets. Our USFDA & En-GMP approved facility in Vapi, Gujarat, is conducting a walk-in recruitment drive on 09 November 2025 for multiple positions across Quality Assurance, Quality Control, Production, Validation, and Warehouse departments. Join a dynamic, growth-oriented organization recognized for innovation, regulatory compliance, and employee development.
Company Overview
V-Ensure Pharma Technologies Pvt. Ltd. is a leading pharmaceutical company focused on research, development, and manufacturing of high-quality oral solid dosage forms. Our Vapi facility strictly follows USFDA and European GMP standards, ensuring compliance with global regulations. With a culture centered on professional growth and innovation, V-Ensure Pharma Technologies empowers its employees to contribute meaningfully to healthcare advancements and global patient safety.
Job Roles & Responsibilities
Quality Assurance (QA)
Positions: Officer / Sr. Officer / Executive
Sections: ALQA, Finished Products, Stability, Method Validation Lab, QA/GLP Documentation Control
Qualifications: MSc / B Pharm / M.Pharm
Experience: 2–6 Years
Key Responsibilities:
-
Review method validation, verification, and stability protocols.
-
Evaluate raw material (RM) and packaging material (PM) specifications, MOAs, and finished product COA.
-
Hands-on experience in QC testing of release and stability samples.
-
Analyze electronic data, audit trails, and integrations.
-
Conduct process validation, vendor qualification, risk assessment, hatch record review, and cleaning validation.
Validation / IPQA
Positions: Officer / Sr. Officer / Executive
Qualifications: MSc / B Pharm / M.Pharm
Experience: 2–7 Years
Key Responsibilities:
-
Execute in-process checks, line clearance, and batch sampling.
-
Ensure compliance with GDP and GMP standards.
-
Perform analysis of RM, PM, FP, and IP stability.
-
Operate sophisticated instruments such as HPLC, GC, UV, FTIR, Dissolution.
Quality Control Analytical Lab
Positions: Officer / Executive
Qualifications: BSc / MSc / B Pharm
Experience: 2–7 Years
Key Responsibilities:
-
Conduct raw material, stability, and finished product testing.
-
Execute analytical method validation and technology transfer.
-
Operate instruments including HPLC, GC, UV, FTIR, Dissolution systems.
Warehouse
Positions: Officer / Sr. Officer / Executive
Qualifications: Any Graduate
Experience: 3–5 Years
Key Responsibilities:
-
Monitor temperature and RH in storage areas.
-
Manage SOP revisions, change control, CAPA, deviations, retest, and rejected materials.
-
Oversee invoice processing and QMS documentation.
Production / Manufacturing
Positions: Operator / Sr. Operator / Officer / Sr. Officer
Qualifications: D.Pharm / Diploma / B Pharm / MSc / M.Pharm
Experience: 2–8 Years
Key Responsibilities:
-
Operate compression, capsule filling, blister packing, granulation, and bottle packing machines.
-
Maintain production area logbooks and documentation.
-
Execute method verification, tech transfer, and analytical testing.
-
Ensure adherence to SOPs, GMP, and QMS standards.
Walk-In Drive Details
-
Date: 09 November 2025 (Sunday)
-
Time: 9:00 AM to 5:00 PM
-
Venue: Hotel Woodland, N.H. No. 48, Near Tejpal Motors, Balitha, Vapi – 396191, Gujarat
-
Documents to Bring: Resume, Passport-size Photo, Last Increment Letter, 3 Months Salary Slips
Note: Candidates with experience in regulated pharma environments, GMP/GLP documentation, and USFDA/EN-GMP facilities will be preferred.
Key Skills & Experience Required
-
Hands-on experience in QA, QC, Production, or Validation in regulated pharma plants.
-
Proficiency in HPLC, GC, UV, FTIR, Dissolution apparatus.
-
Knowledge of GMP, GLP, GDP, and QMS compliance.
-
Ability to manage SOPs, CAPA, change control, and deviation documentation.
-
Strong attention to detail, teamwork, and analytical thinking.
Benefits & Growth Opportunities
-
Career development in a USFDA & En-GMP approved facility.
-
Exposure to regulated domestic and international markets.
-
Hands-on experience with modern pharmaceutical equipment.
-
Work in a professional, collaborative, and growth-oriented culture.
FAQs
Q1: What documents are required for the walk-in?
A: Resume, Passport-size Photo, Last Increment Letter, 3 Months Salary Slips.
Q2: Who will be preferred?
A: Candidates with experience in USFDA/EN-GMP facilities and regulated pharma plants.
Q3: Is prior experience mandatory?
A: Yes, 2–8 years depending on the role.
Q4: Are multiple vacancies available?
A: Yes, across QA, QC, Production, Validation, and Warehouse departments.
V-Ensure Pharma Technologies Walkin
| Category | Details |
|---|---|
| Company | V-Ensure Pharma Technologies Pvt. Ltd. – USFDA & En-GMP approved |
| Vacancies | Multiple across QA, QC, Production, Validation, Warehouse |
| Required Education | MSc, B Pharm, M.Pharm, BSc, D.Pharm, Diploma, Any Graduate |
| Experience | 2–8 Years depending on role |