BiomatiQ Hiring Production,QA, Engineering,R&D

- Company Overview
- Job Roles & Responsibilities
- Eligibility / Qualifications
- Location & Salary
- Application Process
- Why Join BiomatiQ?
- SEO Keyword Suggestions (Integrated Naturally)
- FAQs
- Summary Table
Executive, Technician & R&D Openings – BiomatiQ Hyderabad (M.Sc/B.Pharm)
Apply for Production, QA, Engineering, and R&D roles at BiomatiQ Hyderabad. Openings for M.Sc/B.Pharm candidates with 0–15 years experience.
BiomatiQ Private Limited, a growing pharmaceutical company located in Hyderabad, Telangana, invites talented professionals and fresh science graduates to join its expanding operations. With an unwavering commitment to quality, innovation, and regulatory excellence, BiomatiQ provides an empowering environment where expertise meets opportunity.
Whether you’re an experienced professional in Quality Assurance or an ambitious fresher in R&D, this is your chance to build a rewarding career with a trusted name in the pharma industry.
Company Overview
BiomatiQ Private Limited is a reputed pharmaceutical manufacturer specializing in sterile manufacturing and microbiological testing. Located in the TGIIC Industrial Park, Hyderabad, the company adheres to strict international quality standards while offering end-to-end solutions in sterile production, research, and quality management.
With a vision to advance healthcare through superior quality and compliance, BiomatiQ focuses on sustainable production practices and continuous learning. Its team-centric approach ensures growth, innovation, and professional development for every employee.
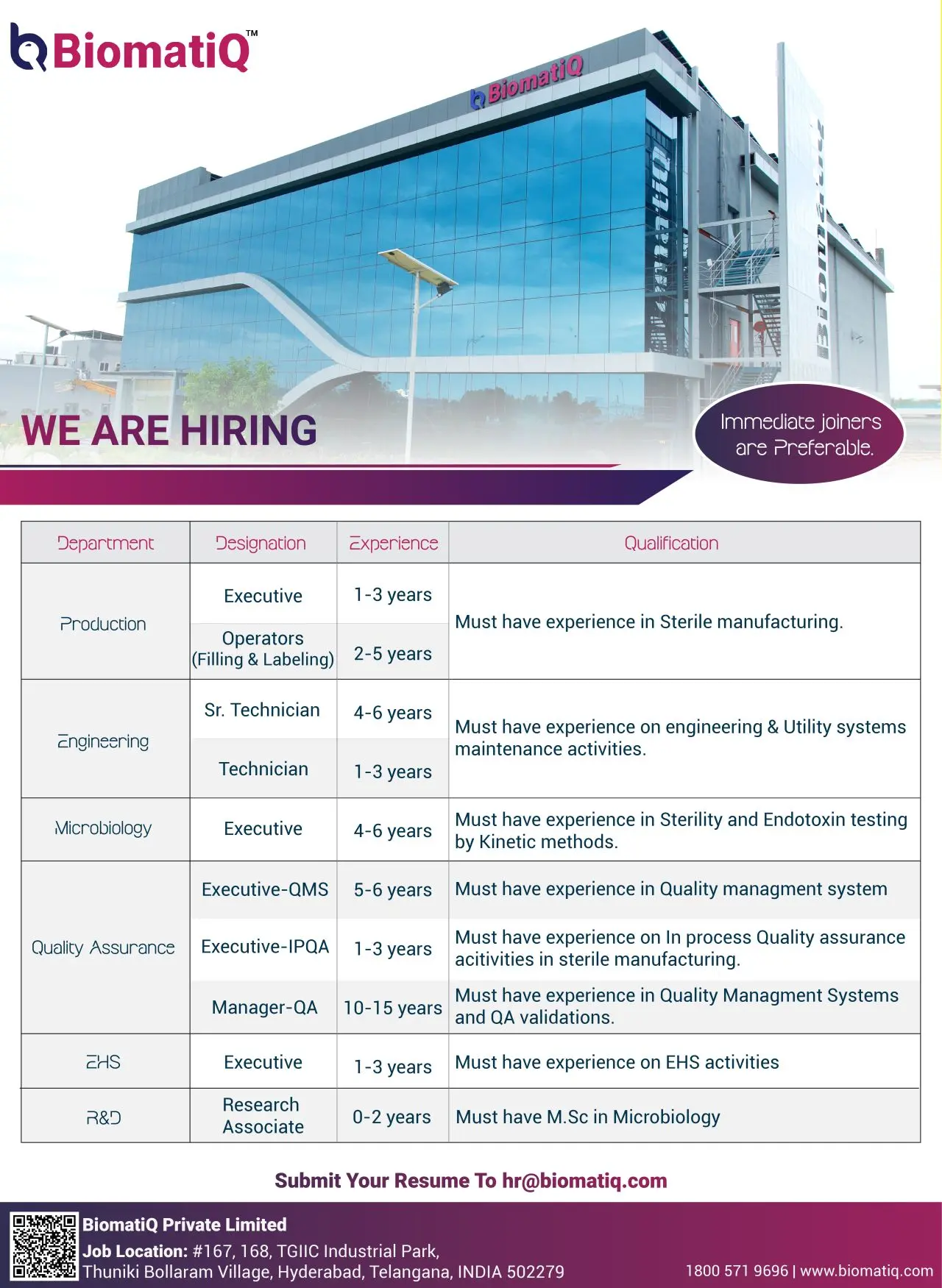
Job Roles & Responsibilities
BiomatiQ is hiring across multiple departments for various levels of experience. Immediate joiners are highly preferred.
Production Department
- Designation: Executive / Operator (Filling & Labeling)
- Experience: 1–3 years
- Responsibilities:
- Perform sterile manufacturing and filling operations.
- Ensure adherence to GMP and production standards.
- Handle labeling and batch documentation.
- Required Skills: Hands-on experience in sterile production and knowledge of aseptic techniques.
Engineering Department
- Designation: Senior Technician / Technician
- Experience: 2–6 years
- Responsibilities:
- Maintenance of engineering utilities and systems.
- Ensure smooth functioning of HVAC, compressors, and water systems.
- Support preventive maintenance and calibration activities.
- Required Skills: Strong understanding of utilities and mechanical systems in pharma setups.
Microbiology Department
- Designation: Executive
- Experience: 4–6 years
- Responsibilities:
- Conduct sterility and endotoxin testing using kinetic methods.
- Maintain microbiological documentation and reports.
- Adhere to GLP and lab safety protocols.
- Required Skills: Expertise in sterility assurance, media preparation, and microbial testing.
Quality Assurance (QA)
- Designation: Executive-IPQA / Manager-QA / Executive-QMS
- Experience: 1–15 years (depending on role)
- Responsibilities:
- Oversee in-process quality assurance in sterile manufacturing.
- Manage Quality Management Systems and validation processes.
- Conduct audits, review CAPA, and ensure regulatory compliance.
- Required Skills: Strong knowledge of QA principles, documentation, and cGMP compliance.
CHS Department (Corporate Health & Safety)
- Designation: Executive
- Experience: 1–3 years
- Responsibilities:
- Manage EHS activities and ensure a safe workplace.
- Conduct safety audits and implement corrective actions.
- Required Skills: Experience in occupational health & safety compliance.
Research & Development (R&D)
- Designation: Research Associate
- Experience: 0–2 years
- Responsibilities:
- Conduct microbiology research experiments.
- Support new product development and analytical method validation.
- Prepare and maintain lab records.
- Qualification: M.Sc. in Microbiology
Eligibility / Qualifications
- Production: B.Pharm, M.Pharm, or Diploma in Pharmacy with sterile manufacturing experience.
- Engineering: ITI, Diploma, or B.Tech in Mechanical/Electrical Engineering.
- Microbiology: M.Sc. Microbiology, Biotechnology.
- Quality Assurance: B.Pharm/M.Pharm with QA specialization.
- R&D: M.Sc. in Microbiology or Life Sciences.
- CHS: Diploma/Degree in Environmental Health, Industrial Safety, or related field.
Preferred: Candidates with experience in sterile formulations, injectable manufacturing, and pharma utilities maintenance.
Location & Salary
- Location: Plot No. 167–168, TGIIC Industrial Park, Thuniki Bollaram Village, Hyderabad, Telangana – 502279
- Salary: Competitive and based on industry standards (negotiable as per experience).
- Employment Type: Full-time, permanent
Application Process
Interested candidates can apply directly by sharing their updated resumes to:
Email: hr@biomatiq.com
Website: www.biomatiq.com
Apply before November 15, 2025 to secure your spot and join one of Hyderabad’s most promising pharma teams!
For more details, contact:1800 571 9696
Why Join BiomatiQ?
- Opportunity to work in a world-class sterile manufacturing setup.
- Exposure to modern microbiological and analytical techniques.
- Career progression through training and performance-based growth.
- Strong culture of compliance, teamwork, and safety.
- Be part of a growing organization contributing to global healthcare.
SEO Keyword Suggestions (Integrated Naturally)
Pharma jobs in Hyderabad, M.Sc Microbiology jobs, sterile production vacancies, QA jobs in pharma, engineering technician pharma jobs, Hyderabad pharma openings, pharmaceutical R&D jobs, microbiologist positions in Hyderabad.
FAQs
1. What qualifications are required to apply for BiomatiQ jobs?
Candidates must hold relevant degrees such as B.Pharm, M.Pharm, or M.Sc. in Microbiology or Life Sciences depending on the department.
2. Is prior experience mandatory?
Experience is required for most roles except R&D, where freshers with M.Sc. Microbiology can apply.
3. What is the location of BiomatiQ?
The facility is located at TGIIC Industrial Park, Thuniki Bollaram Village, Hyderabad.
4. How can I apply?
Submit your CV via email to hr@biomatiq.com mentioning the position in the subject line.
5. What are the career growth opportunities?
BiomatiQ provides continuous learning, exposure to sterile manufacturing, and performance-based promotions.
Summary Table
| Category | Details |
|---|---|
| Company | BiomatiQ Private Limited |
| Vacancies | Executive, Technician, Manager, Research Associate roles across Production, QA, Microbiology, R&D, CHS, and Engineering |
| Required Education | B.Pharm, M.Pharm, M.Sc. Microbiology, Diploma, B.Tech, ITI |
| Experience | 0–15 years (depending on role) |
| Job Location | Hyderabad, Telangana |
| Application Email | hr@biomatiq.com |
| Website | www.biomatiq.com |
| Contact Number | 1800 571 9696 |
To apply for this job email your details to hr@biomatiq.com


