Laurus Labs walk-in API, Formulation, EHS

- Company Overview
- Job Role & Responsibilities
- Analytical Development – API / CDMO / Peptide
- Process Development – API / CDMO / Peptide
- Manufacturing – API
- Manufacturing – Formulation OSD (Production & Packing)
- EHS – Safety & Environment
- Eligibility / Qualifications
- Experience & Positions
- Job Location & Interview Schedule
- Application Process
- Why Choose Laurus Labs?
- Frequently Asked Questions (FAQs)
Laurus Labs Walk-in Interviews – M.Sc, M.Pharm, B.Sc | Vizag, Hyderabad
Laurus Labs walk-in interviews for API, Formulation, EHS roles. M.Sc, M.Pharm, B.Sc eligible. Multiple vacancies in Vizag & Hyderabad.
Laurus Labs, one of India’s leading pharmaceutical and CDMO companies, is conducting large-scale walk-in interviews for its API, Formulation, Analytical Development, Process Development, Manufacturing, and EHS departments. These walk-in drives are open for experienced pharma and chemistry professionals looking to build long-term careers in regulated pharmaceutical manufacturing and research environments. The interviews will be conducted across Visakhapatnam and Hyderabad locations on multiple dates, offering candidates flexibility and direct access to hiring teams.
Known for its strong presence in API manufacturing, CDMO services, peptides, and finished dosage formulations, Laurus Labs continues to expand its operational and R&D capabilities. This hiring initiative supports ongoing growth across production, development, safety, and quality-driven operations. Candidates with hands-on pharmaceutical industry experience will be given preference, making this an excellent opportunity for professionals seeking stability, career progression, and exposure to global regulatory standards.
Company Overview
Laurus Labs is a globally recognized pharmaceutical and biotechnology company with a strong footprint in APIs, formulations, CDMO services, and specialty ingredients. With state-of-the-art manufacturing facilities and a robust research ecosystem, the company supplies to regulated and emerging markets worldwide. Laurus Labs is known for its compliance-driven culture, strong EHS practices, and continuous investment in people, technology, and sustainability.
Operating from key pharma hubs such as Visakhapatnam and Hyderabad, Laurus Labs provides employees with exposure to large-scale pharmaceutical manufacturing, analytical research, process optimization, and formulation development. The company’s consistent growth and expanding product portfolio make it a trusted employer for chemistry and pharmacy professionals.
Job Role & Responsibilities
Analytical Development – API / CDMO / Peptide
Professionals in analytical development will support method development, validation, and analytical troubleshooting for APIs and peptide-based products.
Responsibilities include:
- Analytical method development and validation
- Stability studies and impurity profiling
- Support for regulatory submissions
- Instrument handling such as HPLC, GC, and related techniques
Process Development – API / CDMO / Peptide
Process development associates and executives will focus on route scouting, process optimization, and scale-up activities.
Responsibilities include:
- Process optimization and cost reduction
- Technology transfer to manufacturing
- Supporting pilot and commercial scale batches
- Documentation for development and scale-up
Manufacturing – API
Manufacturing professionals will be responsible for executing API production activities under cGMP conditions.
Responsibilities include:
- API batch manufacturing operations
- Equipment handling and troubleshooting
- Compliance with SOPs and safety norms
- Documentation and batch record maintenance
Manufacturing – Formulation OSD (Production & Packing)
Roles in formulation manufacturing cover both production and packing operations for oral solid dosage forms.
Responsibilities include:
- Tablet and capsule manufacturing
- Packing line operations
- Adherence to GMP and safety standards
- Coordination with QA and QC teams
EHS – Safety & Environment
EHS professionals will support workplace safety, environmental compliance, and sustainability initiatives.
Responsibilities include:
- Implementing EHS policies and procedures
- Conducting safety audits and risk assessments
- Environmental monitoring and compliance
- Training employees on safety practices
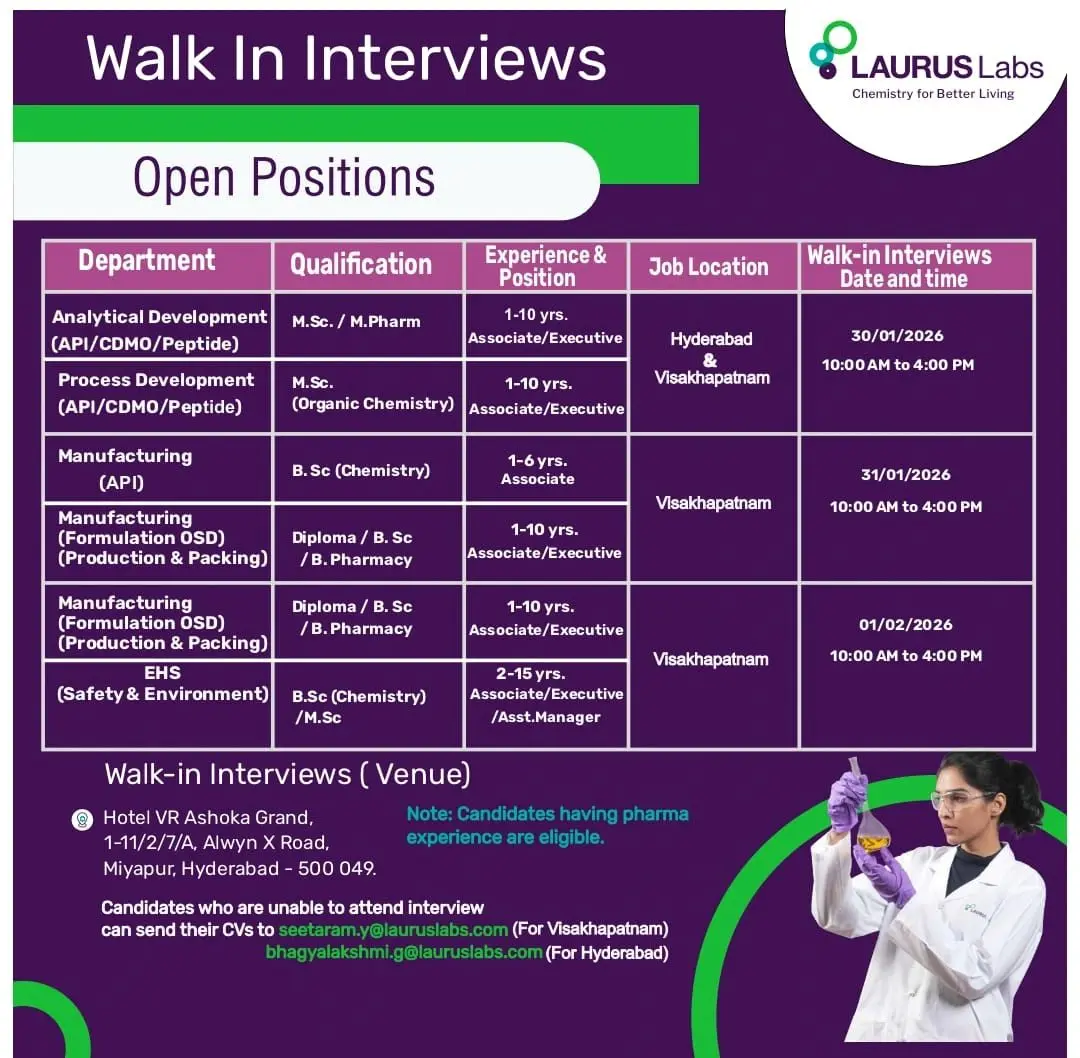
Eligibility / Qualifications
The eligibility criteria vary by department:
- Analytical Development: M.Sc, M.Pharm
- Process Development: M.Sc (Organic Chemistry)
- Manufacturing API: B.Sc (Chemistry)
- Manufacturing Formulation (Production & Packing): Diploma, B.Sc, B.Pharmacy
- EHS (Safety & Environment): B.Sc (Chemistry), M.Sc
Relevant courses include: M.Sc Chemistry, M.Sc Organic Chemistry, M.Sc Pharmaceutical Chemistry, M.Pharm, B.Sc Chemistry, Diploma in Pharmacy, B.Pharmacy.
Experience & Positions
- Analytical Development: 1–10 years | Associate / Executive
- Process Development: 1–10 years | Associate / Executive
- Manufacturing API: 1–6 years | Associate
- Manufacturing Formulation OSD: 1–10 years | Associate / Executive
- EHS: 2–15 years | Associate / Executive / Assistant Manager
Candidates must have prior pharmaceutical industry experience.
Job Location & Interview Schedule
Job Locations:
- Visakhapatnam
- Hyderabad
Walk-in Interview Dates & Timings:
- 30 January 2026 | 10:00 AM – 4:00 PM | Visakhapatnam
- 31 January 2026 | 10:00 AM – 4:00 PM | Hyderabad
- 01 February 2026 | 10:00 AM – 4:00 PM | Hyderabad
Interview Venue:
Hotel VR Ashoka Grand,
1-11/2/7/A, Alwyn X Road,
Miyapur, Hyderabad – 500049
Application Process
Candidates who are unable to attend the walk-in interviews can share their updated resumes via email:
- Visakhapatnam roles: seetararn.y@lauruslabs.com
- Hyderabad roles: bhagyalakshmi.g@lauruslabs.com
Candidates are advised to mention the department and preferred location clearly in the email subject line.
Why Choose Laurus Labs?
- Global pharmaceutical and CDMO exposure
- Strong compliance and EHS culture
- Career growth across API and formulation domains
- Opportunities in development, manufacturing, and safety
- Stable organization with long-term vision
Frequently Asked Questions (FAQs)
Q1: Is this hiring only through walk-in interviews?
No. Candidates who cannot attend the walk-in can apply via email.
Q2: Are freshers eligible for these roles?
These roles are primarily for candidates with prior pharma industry experience.
Q3: What qualifications are accepted?
M.Sc, M.Pharm, B.Sc Chemistry, Diploma, and B.Pharmacy depending on the department.
Q4: Are both API and formulation roles available?
Yes. Openings are available across API, CDMO, peptide, and formulation manufacturing.
Q5: What locations are available?
Roles are available in Visakhapatnam and Hyderabad.
| Company | Laurus Labs |
|---|---|
| Vacancies | Analytical Development Associate/Executive, Process Development Associate/Executive, Manufacturing API Associate, Formulation OSD Associate/Executive, EHS Associate/Executive/Assistant Manager |
| Required Education | M.Sc, M.Pharm, M.Sc Organic Chemistry, B.Sc Chemistry, Diploma, B.Pharmacy |
| Experience | 1–15 years depending on role |
To apply for this job email your details to bhagyalakshmi.g@lauruslabs.com


