Ipca Laboratories Walk-In Engineering, Production, Qa, Apprentice

- Company Overview
- Job Role & Responsibilities
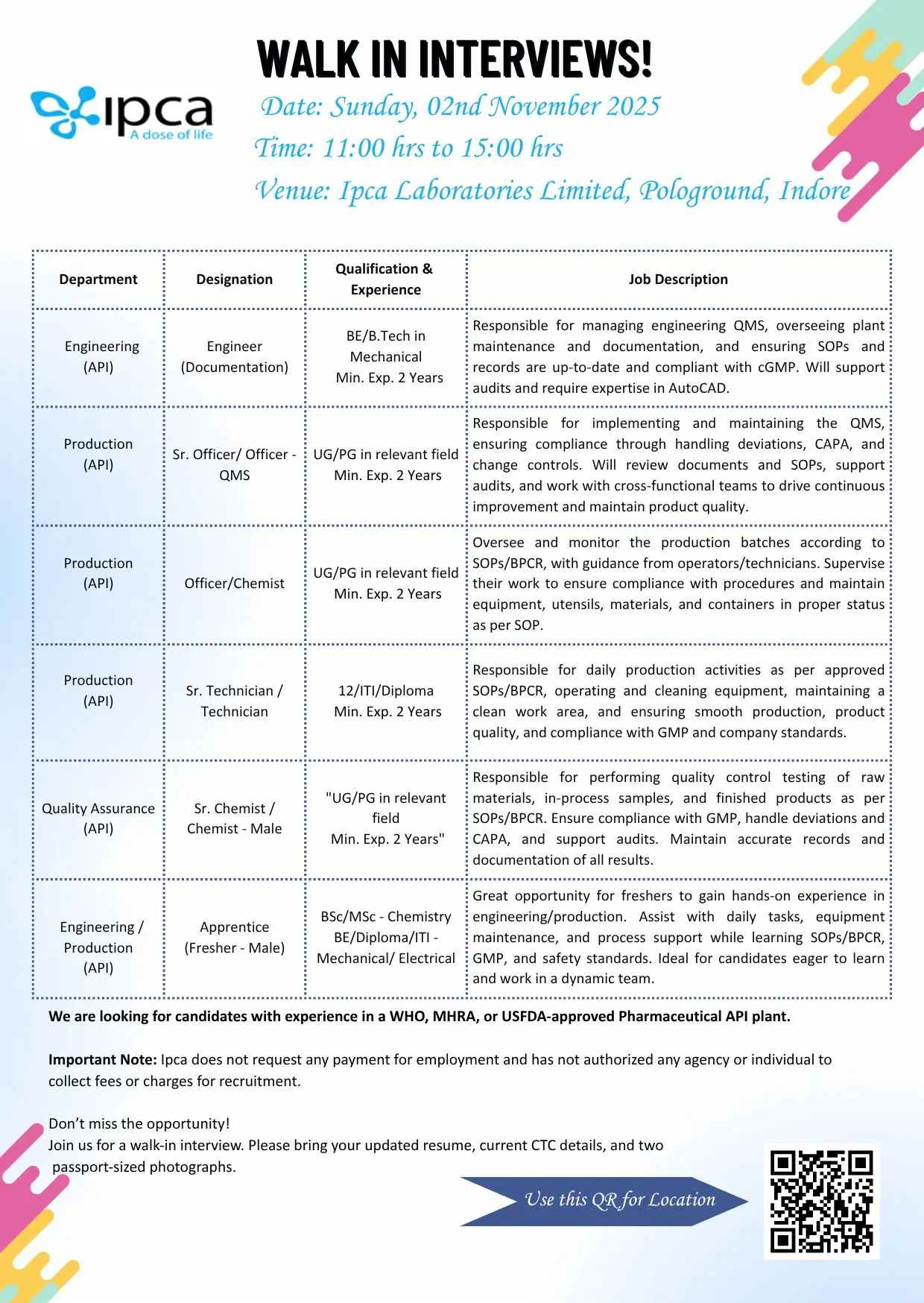
- 1. Engineering (API) – Engineer (Documentation)
- 2. Production (API) – Sr. Officer / Officer QMS
- 3. Production (API) – Officer / Chemist
- 4. Production (API) – Sr. Technician / Technician
- 5. Quality Assurance (API) – Sr. Chemist / Chemist (Male)
- 6. Engineering / Production (API) – Apprentice (Fresher – Male)
- Eligibility / Qualifications
- Location & Walk-In Details
- Application Process
- Why Join Ipca Laboratories?
- FAQs About Ipca Laboratories Walk-In 2025
- Apply Before It’s Too Late!
- Summary Table
B.Pharm, ITI, Diploma Openings at Ipca Laboratories – Indore Walk-In 2025
Multiple pharma vacancies for B.Pharm, ITI, Diploma candidates at Ipca Laboratories, Indore. Attend the walk-in interview on 2nd Nov 2025.
Ipca Laboratories Limited, one of India’s leading pharmaceutical manufacturers, is inviting skilled professionals and freshers for multiple openings in its API Division at the Pologround, Indore Plant. The walk-in interviews are scheduled for Sunday, 2nd November 2025, from 11:00 AM to 3:00 PM. This is a golden opportunity for candidates seeking growth in engineering, production, and quality roles within a WHO, MHRA, and USFDA-approved pharmaceutical plant.
Ipca Laboratories has been at the forefront of healthcare innovation for over six decades, contributing to the global supply of Active Pharmaceutical Ingredients (APIs) and formulations. The company’s unwavering commitment to quality, research, and regulatory compliance makes it one of the most trusted names in the pharmaceutical industry.
Company Overview
Founded in 1949, Ipca Laboratories Ltd. is a globally recognized pharmaceutical company manufacturing a wide range of APIs and formulations. With operations in over 120 countries, Ipca has achieved multiple regulatory approvals from global health authorities such as the USFDA, MHRA, and WHO. Its Indore plant plays a crucial role in producing high-quality APIs that meet international standards.
Ipca is also a Great Place to Work-Certified™ organization that promotes professional growth, continuous learning, and work-life balance for its employees.
Job Role & Responsibilities
1. Engineering (API) – Engineer (Documentation)
Qualification: BE/B.Tech in Mechanical
Experience: Minimum 2 years
Responsibilities:
- Manage Engineering Quality Management System (QMS) documentation.
- Ensure all SOPs, preventive maintenance records, and cGMP documentation are up-to-date.
- Coordinate with internal audit teams to maintain compliance with GMP.
- Hands-on experience with AutoCAD and plant engineering operations preferred.
2. Production (API) – Sr. Officer / Officer QMS
Qualification: UG/PG in relevant field
Experience: Minimum 2 years
Responsibilities:
- Implement and maintain the Quality Management System (QMS).
- Handle deviations, CAPA, and change controls effectively.
- Conduct document and SOP reviews for continuous compliance.
- Support internal and external audits.
3. Production (API) – Officer / Chemist
Qualification: UG/PG in relevant field
Experience: Minimum 2 years
Responsibilities:
- Supervise production batches and ensure all activities follow SOPs and BPCR.
- Maintain process equipment and cleanliness as per GMP standards.
- Guide technicians and operators during batch production and documentation.
4. Production (API) – Sr. Technician / Technician
Qualification: 12th / ITI / Diploma
Experience: Minimum 2 years
Responsibilities:
- Operate, clean, and maintain production equipment as per approved SOPs.
- Support daily production schedules to ensure uninterrupted batch processing.
- Follow Good Manufacturing Practices (GMP) and maintain documentation accuracy.
5. Quality Assurance (API) – Sr. Chemist / Chemist (Male)
Qualification: UG/PG in relevant field
Experience: Minimum 2 years
Responsibilities:
- Conduct testing of raw materials, in-process, and finished products per SOPs.
- Maintain quality documentation and analytical records.
- Handle deviations, CAPA, and audit-related documentation.
- Ensure compliance with GLP and GMP standards.
6. Engineering / Production (API) – Apprentice (Fresher – Male)
Qualification: B.Sc / M.Sc (Chemistry), BE / Diploma / ITI (Mechanical / Electrical)
Experience: Fresher
Responsibilities:
- Assist senior staff in daily production and maintenance activities.
- Learn SOPs, BPCR, and GMP documentation procedures.
- Gain practical exposure to pharmaceutical manufacturing operations.
- Ideal for candidates looking to start their careers in pharma manufacturing and engineering.
Eligibility / Qualifications
- Candidates must have experience from pharma API plants with WHO, MHRA, or USFDA approvals.
- Freshers applying for Apprentice positions should have relevant qualifications and strong interest in learning pharmaceutical production operations.
- Male candidates preferred for all positions as per operational requirements.
Location & Walk-In Details
Venue: Ipca Laboratories Limited, Pologround, Indore, Madhya Pradesh
Date: Sunday, 2nd November 2025
Time: 11:00 AM to 3:00 PM
Location Map: View on Google Maps
Please bring:
- Updated Resume
- Current CTC details
- Two passport-sized photographs
Contact HR:
Mr. Sanjay Makwana – 8347434234
Mr. Harsh Barot – 9724048678
Mr. Jimit Sharma – 6355124377
Application Process
Interested candidates can attend the walk-in interview directly at the above venue. Ensure you carry all essential documents for verification.
Alternatively, candidates can also share their profiles and resumes to the official HR team for future opportunities through Ipca Laboratories’ career portal or by contacting HR via provided contact numbers.
Important: Ipca Laboratories does not charge any fees for recruitment or authorize third-party agencies for the hiring process.
Why Join Ipca Laboratories?
- Work with a globally recognized pharmaceutical brand known for its innovation and compliance excellence.
- Opportunities for career growth in API production and quality operations.
- Exposure to regulatory frameworks like USFDA, MHRA, and WHO GMP.
- Excellent training environment for freshers and career development for experienced professionals.
FAQs About Ipca Laboratories Walk-In 2025
1. Who can apply for Ipca Laboratories Walk-In Interview 2025?
Candidates with qualifications like B.Pharm, M.Sc, ITI, Diploma, BE/B.Tech and relevant API production or QA experience can apply.
2. Are freshers eligible?
Yes, Apprentice (Fresher) positions are available for B.Sc/M.Sc/BE/Diploma/ITI candidates.
3. What is the location of the walk-in interview?
The walk-in will be held at Ipca Laboratories Limited, Pologround, Indore, Madhya Pradesh.
4. What documents should I carry?
Bring your updated resume, recent photographs, and CTC details.
5. Does Ipca charge any recruitment fees?
No. Ipca Laboratories does not charge any application or recruitment fees.
Apply Before It’s Too Late!
This is your chance to grow your career with a reputed pharmaceutical company.
Attend the walk-in interview on 2nd November 2025 (Sunday) between 11:00 AM – 3:00 PM. Don’t miss out—bring your resume and join the team that’s delivering “A Dose of Life.”
Summary Table
| Company | Ipca Laboratories Limited |
| Vacancies | Engineering, Production, Quality Assurance, Apprentice Roles |
| Required Education | B.Pharm, M.Sc, B.Sc, ITI, Diploma, BE/B.Tech |
| Experience | 0–8 years (Freshers and Experienced) |
| Location | Pologround, Indore |
| Interview Date | 2nd November 2025 |
| Time | 11:00 AM – 3:00 PM |
| Contact | HR – Mr. Sanjay Makwana (8347434234), Mr. Harsh Barot (9724048678), Mr. Jimit Sharma (6355124377) |