BDR Pharmaceuticals Hiring QA & Production API

- Company Overview
- Job Role & Responsibilities
- QA (Compliance) – API
- Production – API
- Eligibility / Qualifications
- Location & Salary
- Application Process
- FAQs
- Summary Table
QA & Production API Hiring | BDR Pharma Luna Plant
BDR Pharmaceuticals hiring QA Compliance (4–5 yrs) and API Production trainees/officers (0–2 yrs) at Luna, Vadodara.
BDR Pharmaceuticals is expanding its API operations and hiring experienced QA Compliance professionals along with freshers and early-career candidates for Production at the Luna plant in Vadodara. These roles suit individuals who want to build their careers in API manufacturing, regulatory compliance, and GMP-driven operations.
Company Overview
BDR Pharmaceuticals is a leading name in the oncology and specialty pharma segment, known for its strong manufacturing capabilities and commitment to high regulatory standards. The Luna API facility supports global markets by producing high-quality intermediates and active ingredients under strict cGMP conditions. The organisation emphasises continuous improvement, regulatory alignment, and technical excellence.
Job Role & Responsibilities
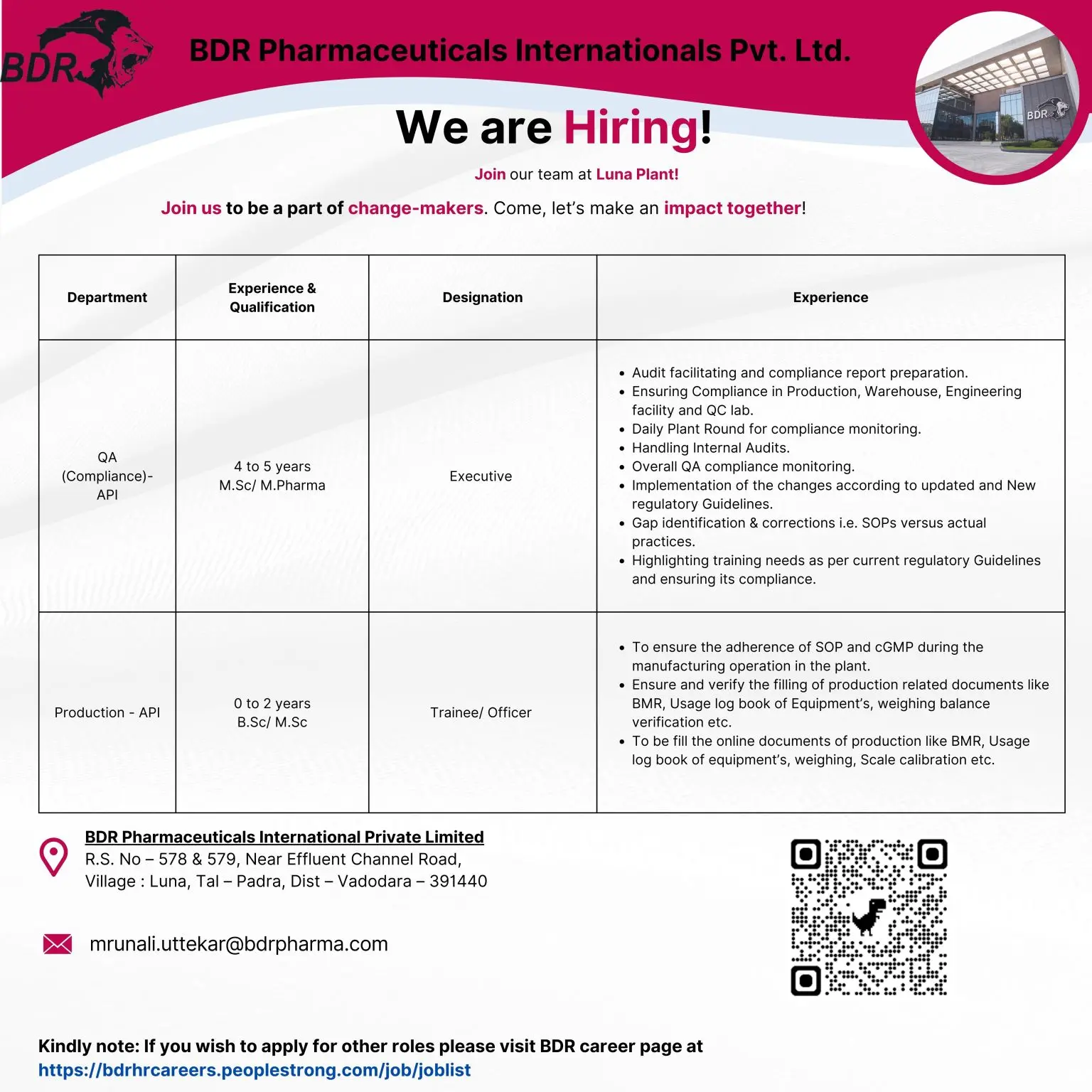
QA (Compliance) – API
Designation: Executive
Qualification: M.Sc / M.Pharm
Experience: 4–5 years
Key Responsibilities:
- Facilitate internal and external audits.
- Prepare compliance reports and review process gaps.
- Monitor compliance in Production, Warehouse, Engineering, and QC.
- Conduct daily plant rounds for GMP adherence.
- Ensure implementation of new and updated regulatory guidelines.
- Identify deviations between SOPs and actual practices and drive corrections.
- Highlight training needs and ensure execution.
- Oversee adherence to cGMP and SOPs during manufacturing.
- Verify and monitor documentation accuracy (BMRs, equipment logbooks, weighing balances, calibrations, etc.).
- Support digital/online documentation systems.
Production – API
Designation: Trainee / Officer
Qualification: B.Sc / M.Sc
Experience: 0–2 years
Key Responsibilities:
- Assist in API manufacturing operations as per SOPs.
- Support batch execution, in-process checks, and documentation.
- Handle equipment usage logs, cleaning, and basic troubleshooting.
- Follow safety practices and maintain production discipline.
- Learn and implement GMP-compliant workflows.
Eligibility / Qualifications
- QA Compliance: M.Sc or M.Pharm with 4–5 years in API QA.
- Production API: B.Sc or M.Sc with 0–2 years.
- Strong understanding of cGMP, documentation, and regulatory expectations.
- Good communication, documentation discipline, and willingness to work in API facilities.
Relevant Courses: M.Sc Organic Chemistry, M.Sc Industrial Chemistry, M.Pharm QA, B.Sc Chemistry, PG Diploma in QA/QC.
Location & Salary
- Work Location: BDR Pharmaceuticals International Pvt. Ltd., R.S. No. 578 & 579, Near Effluent Channel Road, Village Luna, Tal-Padra, Vadodara – 391440.
- Salary: As per company norms; varies by experience and role.
Application Process
Eligible candidates can apply by sharing their CV via email:
For other vacancies, visit the official career page: https://bdrhrcareers.peoplestrong.com/job/joblist
FAQs
1. Can freshers apply?
Yes, freshers can apply for Production API trainee roles.
2. Is QA Compliance open to freshers?
No. Minimum 4 years experience is required.
3. What skills improve selection chances?
Strong GMP knowledge, documentation discipline, and understanding of API processes.
4. Are these roles onsite?
Yes, all positions are based at the Luna plant.
5. Does QA require audit experience?
Yes. Experience in internal audits, compliance checks, and regulatory readiness is preferred.
Summary Table
| Category | Details |
|---|---|
| Company | BDR Pharmaceuticals International Pvt. Ltd. |
| Vacancies | QA Compliance Executive; Production API Trainee/Officer |
| Required Education | M.Sc/M.Pharm (QA), B.Sc/M.Sc (Production) |
| Experience | QA: 4–5 yrs; Production: 0–2 yrs |
To apply for this job email your details to mrunali.uttekar@bdrpharma.com


